International Journal of
eISSN: 2576-4454


Review Article Volume 2 Issue 2
Department of Geosciences and Environment, California State University, USA
Correspondence: Barry J Hibbs, Department of Geosciences and Environment, California State University, USA
Received: October 27, 2017 | Published: April 25, 2018
Citation: Hibbs BJ. Factors in developing salt balances in groundwater basins. Int J Hydro. 2018;2(2):231-238. DOI: 10.15406/ijh.2018.02.00074
This paper discusses criteria and methodology for developing Salt Balance Analysis in Groundwater Basins. The project describes a basic approach to account for geological processes including water/rock and water/soil interactions and mineral dissolution as an in situ source of salt loading to groundwater basins. Salt balance terms are summarized along with factors responsible for the variation of the respective loading terms. Methods of determining total dissolved solids (TDS) are discussed to allow conformity between time series data in long term analysis of salt loading to aquifers, using multiple historical data bases.
The State of California now requires Salt and Nutrient Management Plans (SNMP) to be developed for groundwater basins. The state policy requires plans to be developed for every groundwater basin in California. The required elements of a SNMP include:
Development of SNMPs will lead to a more comprehensive approach to basin water quality management. While the rules described in this paper are germane to recent policy developed in California, USA, the guidelines and issues discussed in this paper can be extended to any basin where salt management issues are relevant. Acceptable loading of salts, nutrients, and other constituents that may adversely affect groundwater quality when recycled water and stormwater are used requires development of accurate water balance terms, knowledge of aquifer properties and processes, and understanding of environmental conditions that modify water balances in groundwater basins. Similarly, loading of natural and anthropogenic sources of salts and nutrients require an understanding of the water balance. Water balance inputs (e.g., interbasin groundwater flow, natural recharge) can enrich or dilute nutrients and salts in groundwater flow systems depending on the specific type of input, the input rate, and the salt or nutrient load contained in the input. The ambient groundwater quality will determine if an input either degrades or improves the water quality in an aquifer, and if the degradation is within acceptable tolerance limits and assimilative capacity. Water balance estimates in a given basin are integral to developing a salt balance and range from well constrained and highly accurate to very suspect estimates that have been developed with anecdotal information. Salt and nutrient assessments must integrate uncertainty to provide a margin of safety based on the reliability of information used to develop accordant water balance and mass transfer assessments. This paper summarizes approaches to estimating the extent of buffering that should be provided for good quality groundwater basins in light of the potential impact of, for example, increased recycled water use and stormwater reuse on groundwater quality. This paper focuses solely on basic aspects of Salt Balance Analysis. Nutrient analysis and nutrient plans will be covered in another paper.
The salt balance
The salt balance for any given land area or soil unit can be expressed by the following equation.1
Where:
Sp, Salt in natural precipitation falling upon the area;
Si, Salt in the irrigation water used in an area
Sr, Residual salts in the soil
Sd, Salt dissolved into solution from soil minerals
Sf, Salt in applied fertilizers or soil amendments
Sc, Salt taken up by or retained on the plant and removed
Sppt, Salt chemically precipitated in the soil
Sdw, Salt in the drainage water from the area
With respect to salt inputs, the salt in natural precipitation, Sp, is usually very small (<5mg/L TD), except near coastal areas where the values can reach at least mentionable values of 10 to 20mg/L TDS.2‒4 Si is a function of the salt concentration in the irrigation water which may be moderate (e.g., 400mg/L TDS) when irrigation source comes from various imported or domestic sources, or may be moderately high (e.g., 1000mg/L TDS) when recycled water is used.5 Sr can be large or small depending on climate, basin hydrological conditions, and land use. Sr is often significant in natural arid landscapes that have seen substantial accumulation of salts in shallow soils, usually due to high evapotranspiration rates combined with little rainfall. Sd is highly variable, depending on type of minerals present in vadose zone and aquifer strata. The presence of soluble marine rocks and detritus in groundwater basins often creates high salt loading to soils and aquifers due to in situ mineral dissolution. Where sediments in aquifers are derived from erosion of igneous or metamorphic rocks in highlands, loading factors due to Sd are usually low. Sf is usually low but can be significant when soils are chemically amended with gypsum and other soil conditioners used in agricultural landscapes. With respect to salt outputs, Sc can be important when light watering is done with sprinklers in aesthetic and recreational landscapes (golf courses, parks, and yards). This leaves evaporative salt dust on plant tissues which may be removed by mowing, runoff, or wind deflation. Sppt can be important where light watering is done in urban and recreational landscapes, but is usually managed in agricultural areas as a result of managed leaching of croplands to protect plant health. Sdw drains into surface water or groundwater. Sdw can be reduced through effective land management strategies.5,6 Several of these salt balance components must be evaluated for their importance and uncertainty. The salt loading from in situ mineral dissolution, Sd is a key evaluation parameter due to its importance in many areas, particularly where sedimentary rock aquifers and detritus are present in formations. Sd is evaluated further in this paper.
In situ mineralogical inputs (Sd)
Sd is highly variable, depending on type of minerals present in vadose zone and aquifer strata. The presence of soluble marine rocks and detritus in groundwater basins often creates high salt loading to soils and aquifers due to in situ mineral dissolution. Where sediments in aquifers are derived from erosion of igneous or metamorphic rocks in highlands, loading factors due to Sd are usually low. Sd and Sr are grouped together below as the principal factors in salt loading in California Basins. In arid regions, significant salt concentration effects occur as plant roots osmotically exclude salts in soil water, leaving behind salt enriched soils. When salts are later leached in soils by precipitation recharge, the leachate eventually reaching groundwater may contain several hundred to a few thousand milligrams per of dissolved salts.7 This is especially common in dry areas where the recharge amounts to only a few millimeters per year. Where wastewater is applied to urban landscapes, the salts are already enriched in the feed water and evapoconcentration effects are amplified. While most land application projects predict accumulation of salts in soil profiles during land application of wastewater and irrigation water, the tacit assumption is that mass balance eventually occurs (salt mass applied to soil profiles equals salt mass lost to runoff, plus leaching fraction lost to deep percolation to groundwater). This is not a strictly valid assumption because precipitation of less soluble ions contained in soil solutions (Ca, HCO3) may lead to permanent loss of soil solutes. These mineral precipitation losses are usually minimal however, accounting for no more than a very few percent of the dissolved mineral content in soil water. If evaporative salts form at land surface, such as the surface salt crust thernardite, the salts may be stripped from the land surface by heavy windstorms. This process might represent a measurable percentage loss of dissolved solids in soil water. About 98% of the salt content of aquifers is due to the presence of common rock forming cations and anions. Common ions include Ca, Mg, Na, K, HCO3, CO3, SO4, and Cl.8 Carbonate (CO3) is not present unless groundwater has pH higher than 8.22. Silica dioxide (SiO2), an inert compound, accounts for part of the dissolved load of aquifers. Silica usually has fairly low solubility and is usually not present in groundwater in concentrations exceeding 10 to 35mg/L. Common rock forming minerals release ions and silica by dissolution reactions, hydrolysis reactions, and oxidation reaction (Table 1). Solubility of specific minerals determines how concentrated these ions become in groundwater. Within groundwater systems, some of the natural salinization develops in aquifers by retention/pickup of ions from the atmosphere during recharge of precipitation and runoff. Usually a more important natural source of dissolved salts is from weathering of the common rock minerals contained in aquifers. The salt loading from in situ mineral dissolution, Sd is a key evaluation parameter due to its importance in many areas, particularly where sedimentary rock aquifers and detritus are present in water bearing formations. Silt and clay bearing rocks (mudstones and shales) often form in anaerobic environments due to high organic matter content.8 Reduced mineral species such as pyrite often forms in these fine textured rocks. When the rocks are eventually exposed by tectonic uplift, conditions can turn from anaerobic to aerobic and pyrite and other reduced minerals can be oxidized. Pyrite oxidation releases sulfate and other ions into subsurface waters, sometimes in rather high concentrations.8
Calcite |
|
Dolomite |
|
Pyroxene |
|
Amphibole |
|
Albite |
|
Anorthite |
|
Gypsum |
|
Halite |
|
Table 1 Common mineral dissolution reactions and products
In several coastal groundwater basins in California, Texas, and elsewhere, shallow aquifers (e.g., 50 to 100 meters deep) often yield good groundwater quality with low salt content and Na, Ca, and HCO3 as the dominant ions. An exception occurs where pollutant sources near land surface, such as agriculture of landfills, have contaminated shallow groundwater by salts. Aquifers at intermediate depths, between 100 and 250 meters, tend to yield older water with more sodium than calcium; primarily due to ion exchange processes on clay surfaces. Waters at intermediate depth often have low TDS. Below intermediate depths, the groundwater is generally affected by salty marine water and connate water enriched in sodium and chloride.9,10 All coastal aquifers are vulnerable to saltwater intrusion when they are heavily pumped. Water that has moved long distances through aquifers and that has had a long residence time often contains high salt content. The more soluble anions such as chloride and sodium are particularly elevated. This is the consequence of the variable saturation states of certain minerals. The Chebotarev Sequence11 describes this evolution of groundwater along flowpaths, undergoing dissolution and precipitation reactions as the groundwater becomes older. The groundwater evolves toward the composition of seawater, as illustrated by the schematic:
Travel Along Flowpath ⇒
Groundwater Age ⇒
In the Chebotarev Sequence, the initial anionic components in dilute, recent groundwater are derived by weathering of common silicate minerals and carbonates, producing bicarbonate. As the water passes further down the flowpath and becomes older, and/or moves slowly through deeper, less permeable strata, the water reaches calcite saturation and bicarbonate does not continue to increase in concentration. More soluble minerals, such as gypsum and halite dissolve and pyrite and other reduced minerals are oxidized. This process produces elevated sulfate and chloride. Eventually sulfate reaches solubility limits and gypsum is precipitated or sulfate is reduced to sulfide gas, forming pyrite in the presence of soluble iron. The latter phenomena happen in deeper anaerobic aquifers. Subsequently, chloride becomes the dominant anion in the evolving groundwater flow system. While the Chebotarev Sequence is an established condition in groundwater basins, it is also known that controls on bulk concentration of natural salts in groundwater are not simply a function of residence times in aquifers, as elucidated by the Chebotarev Sequence. As a covariable to the Chebotarev Sequence, natural salt content also develops as a function of reaction with specific minerals present in the groundwater basin (Table 1). Partial pressure of carbon dioxide in soil atmosphere and water and concentration of H+ ions in soil water and groundwater create additional important controls on natural groundwater quality.
As denoted by the Chebotarev Sequence,11 bicarbonate concentrations do not reach values seen for SO4 and Cl in mature groundwaters. Bicarbonate is often much more concentrated than either sulfate or chloride in very young waters or in aquifer strata containing neither sulfur or chloride bearing minerals. The reverse is usually true in mature groundwaters. Gypsum has a moderate solubility so sulfate can reach natural concentrations of several hundred to several thousand mg/L in groundwater. Sulfate concentration limits may exist because aquifer strata containing mature waters may often be characterized as a reducing type of water. Reducing conditions in aquifers will convert sulfate to hydrogen sulfide and metal sulfide, creating important controls on sulfate concentration. Halite, sylvite, and other chloride bearing minerals have the highest solubility. Chloride can reach many tens of thousands of mg/L in very mature groundwaters.4 When groundwater is contained in aquitards, it often moves slowly and has long residence times. In such cases, the Chebotarev process may develop locally in fine textured strata. In addition, aquitards often contain silt and clay which may contain pyrite, gypsum, and other potentially soluble minerals. When these minerals dissolve, an enriched salt composition develops in the confining units. During pumping or artificial recharge, hydraulic heads may change in the aquifers resulting in unnatural pressure gradients across confining units.12 Saline pore waters are then expelled from the confining units, creating an internal loading of salts to aquifers. It follows that any management activity in a groundwater basin causing rapid head changes can create an internal salinization process in permeable parts of aquifers when saline pore waters are expelled from confining strata. In basin fill, the dissolved solids are dependent on the rock fragments and minerals present in the fill material (Figure 1). The surface area of sediment grains in contact with groundwater is greater than the surface area in contact with rock matrix in igneous and metamorphic rocks, leading to more enhanced potential for water-rock interactions. Often the quality of groundwater in basin fill material approximates that of the source rock, provided that no secondary minerals, such as evaporates, are present in alluvium.
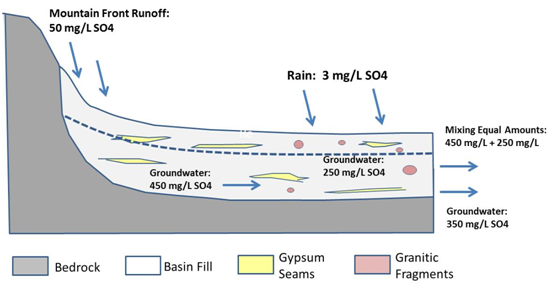
Figure 1 Mixing sources where aquifer strata in a zone contains dominantly sedimentary rock and a mixture of sedimentary rock and granitic fragments due to two different source inputs to basin fill. The salinity inputs are different after the water/soil interactions but reach an averaged zonal value after mixing.
Background to the methodology for developing the salt budget for geologically defined zones is provided in the San Fernando Valley Ground Water Basin as an example of aquifer salinity zonation. Description of methodology is based on the mineralogical sources of salinity; Sd. Striking natural contrasts of salinity in the San Fernando Valley Groundwater Basin (Figure 2) illustrate mineral dissolution factors in salt loading (Sd). The hydrologic boundaries of the groundwater basin encompass the full watershed extent of the Los Angeles River and associated tributaries, upstream of the confluence of the Los Angeles River and Arroyo Seco (Figure 2). The groundwater basin includes the water-bearing sediments beneath the San Fernando Valley, Tujunga Valley, and Browns Canyon, as well as the alluvial areas surrounding the Verdugo Mountains near La Crescenta and Eagle Rock (Figure 2). The basin is bounded on the north and northwest by the Santa Susana Mountains, on the north and northeast by the San Gabriel Mountains, on the southeast by the San Rafael Hills and San Repetto Hills, on the south by the Santa Monica Mountains and Chalk Hills, and on the west by the Simi Hills.13 The Verdugo Mountains separate the Verdugo Basin from the San Fernando Basin. The valley is drained by the Los Angeles River and its confluent tributaries.
The water-bearing sediments consist primarily of the lower Pleistocene Saugus Formation as well as Pleistocene and Holocene age alluvium.14 The groundwater in this basin is mainly unconfined. Some confinement occurs within the Saugus Formation in the western part of the basin.14 Recharge of the basin comes from imported and natural sources, and spreading of imported water and runoff occurs in the Pacoima, Tujunga, and Hansen Spreading Grounds. Runoff comes from precipitation falling on surrounding mountains and urban areas. Water flowing in surface washes recharges the basin directly. Due to the origin of soluble alluvium fragments from the erosion of sedimentary outcrops and bedrock, the western and southwestern parts of the basin are noted for higher TDS of a calcium-sulfate-bicarbonate type. The alluvium in northeastern and southeastern parts of the basin are dominantly derived from granitic and other igneous rocks in the San Gabriel Mountains and are characterized by lower TDS and a calcium-bicarbonate type water.15,16 TDS range from 326 to 615 mg/L in active wells to the east, but wells (many no longer used) in the western part of basin historically produced TDS in excess of 1000mg/L. Much of the salinity has been demonstrated to develop due to water/rock and water/soil interactions (geological sources) in aquifer and vadose zone strata. Early investigations revealed the important controls of geology on predevelopment salt concentration arising from the water-rock interactions with aquifer strata of different geological origin. One of the first accounts is identified in Bulletin 40, Quality of Irrigation Waters by California Division of Water Resources.17 In this document variable salinities in the San Fernando Valley Basin are described:
“The water under the northeast side of the Valley is essentially different in quality from that under the southwest side, particularly in sulphate content, and the line of demarcation between the two is sharp………….In a previous paragraph is a reference to a sharp line of demarcation between the high sulphate waters under the southwest side of the valley and the low sulphate waters under the northwest side. The contrast in the conditions on the two sides of the line is shown in the following table, No. 5 [editorial note: original table is modified as Table 2 in this paper]. It is to be inferred that the underground water derived from the Santa Monica Mountains becomes charged with sulphate from that source, [i.e., editorial note: the rocks and sediments from the mountains] while the water derived from the northeast, including that imported from Owens Valley, through the Los Angeles Aqueduct, is not so charged”.
The California State Water Rights Board14 enumerates how geological units vary and are related to salinity contrasts in the basin:
Southwest side |
Northeast side |
||||
Well number |
Specific conductance (mS/cm) |
Sulphate content (mg/L) |
Well number |
Specific conductance (mS/cm) |
Sulphate content (mg/L) |
26a |
1580 |
601 |
31a |
490 |
39 |
78f |
1500 |
598 |
80h |
431 |
35 |
81k |
1320 |
408 |
84f |
431 |
30 |
88g |
1100 |
310 |
88 |
573 |
61 |
Table 2 Comparison of the conductance and sulphate content of adjacent wells on two sides of a line in the san fernando valley
(Modified from California division of water resources, 1933)
“Recent deposits east of Pacoima Wash and north of the Los Angeles River consist of predominantly coarse accumulations of boulders, gravels, and sands in the form of coalescing alluvial fans derived primarily from basement complex sources. West of Pacoima Wash and south of the Los Angeles River, the sediments are derived primarily from sedimentary rocks and are finer grained and deposited in much the same manner as the underlying older alluvium”.
Further information, including a set of salinity contour maps; define the sharp contrasts in salt concentration as a function of lithological variability. Setmir18 elaborates:
“The majority of streams in the western part of the San Fernando subbasin are underlain by sedimentary rocks which contain gypsum, thereby producing runoff high in calcium sulfate. The streams in the eastern part of the subbasin, with runoff from areas that are granitic (from basement complex) produce a predominantly calcium carbonate water…..The character of groundwater from the major water-bearing formations is of two general types, each reflecting the composition of the surface-water runoff in the area. In the western part of the valley, it is calcium-sulfate-bicarbonate in character, whereas in the eastern part, including the Sylmar subbasin, it is calcium-bicarbonate….. wells in the western end of the San Fernando subbasin have excess concentrations of sulfate”.
The published sources demonstrate how geological materials control the predevelopment/natural salinity in the San Fernando Valley Basin Aquifer, with significant spatial variations across the basin. Natural geological dissolution sources need to be enumerated in a salt budget in all influent waters coming in contact with these geologic units, particularly where they contain soluble minerals. This is essential in developing a realistic Salt Balance Analysis.
Mapping out predevelopment water quality is done in steps. Published historical water quality maps are beneficial. If such maps are not available then other steps must be taken to do the assessment. The first step is to locate early or predevelopment groundwater quality data across the basin. If predevelopment water quality data is relatively homogeneous across the basin than the selection of “zones” is determined based on other criteria, such as subdivision by hydrologic subbasins. If lithology and predevelopment water quality are highly variable, as found in the San Fernando Basin, then the methodology we summarize should be considered in developing a Salt Balance Analysis. To map out water quality zones, it is necessary to delineate aquifer strata derived from their geologic source rocks. Examination of test hole information described by a geologist is needed. Useful information includes textures, mineralogy, and description of any secondary mineral cements or evaporites that may be present. Another important step is to identify predevelopment or early development water quality across the basin. It is especially important to identify predevelopment salinity zones. Once predevelopment water quality zones are mapped out the source(s) of recharge water and their mixing percentages must be accounted for. Within a particular zone, water quality derives from different mixing sources. As an example, a simple schematic mixing diagram is used in demonstration (Figure 1). In this example, the aquifer salinity zone may have variable lithologies that produce groundwater of different salinity within the specific zone. In the example shown (Figure 1), the aquifer includes a mixture of gypsum and granitic fragments basinward of the mountain front. These are derived from different rock sources washed in from separate drainages or from transitions in depositional processes when the basin fill was forming. In such an example it is logical that direct recharge from precipitation in the basinward part of the aquifer will have a lower salinity than recharge from mountain front percolating solely through sedimentary rock detritus containing gypsum (the mountain front area).
Though this example presents a simplified case of equal mixing of mountain front runoff and direct precipitation recharge it illustrates an important point. The equal parts of salinity of each parcel of recharge are attained for the final zone concentration (250mg/L + 450mg = 350mg/L). The final predevelopment salinity in this zone is calculated as 350mg/L, due to equal mixing between the two sources in predevelopment times. While this is a very simplified case for illustration purposes it demonstrates how predevelopment salinity of a zone may arise due to mixing of waters of different sources and different salinity. Some zones are very saline, some are very dilute. However, they provide background water quality to which land use changes provide loading or dilution of salts in an aquifer zone. Once predevelopment salinity values are estimated, it is possible to assess changes that have occurred in aquifer zones as a result of loading factors listed in the salt balance equation.
Geological input for mountain front recharge, which replenishes the aquifer zone by partial flow through bedrock, might be considered to be about 25% more saline than replenishment of the zone by recharge by precipitation and delivered water along the basin floor. This is because the minerals in the bedrock formations are assumed to be less weathered and more soluble than basin floor sediments that are usually derived from bedrock source material containing weathered erosional fragments with fewer soluble minerals. Spread water recharge generally percolates through thin vadose zones where the underlying aquifer contains permeable low solubility sediments. Spreading basins are more regularly flushed by percolating waters than ordinary basin floor areas. It can therefore be assumed that less salinity of geologic origin is picked up by percolating recharge waters beneath spreading basins. These generalizations are germane to the zones in a basin. The methodology for establishing ion and TDS values for zones requires an averaging function for water quality across the entire zone at a fixed point in time as initial conditions. The calculated input values can be developed for predevelopment times, for modern times, or for future projections based on projected test scenarios. Once established, a salinity parameter is taken as a single bulk value for the entire zone prescribed as an initial condition.
An individual basin may often be discretized into many zones based on salinity differences across the basin. Lateral zones may also be separated into multilayer stacked systems to cover possible vertical changes of properties or salinity in the zone. Each zone requires a separate water budget and each requires input salinity values for all water balance flow terms. It is cumbersome to deal with an excessive number of zones, so the analyst is advised to balance the number of zones for an aquifer based on the complexity of the system and density of subarea control data. If the systems are very complex the selection criteria for establishing zones should be based on transitions in the aquifer allowing subarea water and salinity budgets to be developed. The initial conditions for salinity must be evaluated with groundwater salinity data taken from water wells. The initial salinity input value is homogenized across the entire zone. The value may not be representative of overall zone salinity if data used to develop the estimate is taken only from high capacity production wells. Frequently high capacity wells tap aquifers at depths where the highest quality groundwater (i.e., the most dilute groundwater) is produced. The shallowest and sometimes the deepest parts of the aquifer contain more saline groundwater; sometimes much more saline groundwater. It is therefore incumbent on the analyst to average salinity values across the full volume of the aquifer zone in order to provide a realistic “bulk” salinity value for the zone. Where individual ions (e.g., sulfate and chloride) and TDS are calculated, this must be done while maintaining salinity values that plausibly reflect cation/anion balance. The use of a few groundwater samples collected from similar target depths are usually not sufficient for estimating the bulk average salinity value in the zone. In such cases, other averaging schemes must be chosen. Downhole electric log interpretations is one possible avenue to consider for calculating initial value of TDS. Generally borehole geophysical techniques are not effective for calculating values of individual ions however.
Cation-anion balance is also germane to the task of developing salt balances in groundwater basins. As will be demonstrated, an unrealistic input concentration of TDS and Cl in a Salt Balance Analysis would be, for example, 100 mg/L TDS and 80 mg/L Cl in recharge input water. A realistic analysis derives from taking into account the cation/anion balance and condition of electrical neutrality that is a fundamental property of groundwater. In Salt Balance Analysis the assumption of cation/anion electrical neutrality is used repeatedly, and the summary is presented below to demonstrate a necessary specification of a multi-constituent salt balance. Many salt balances performed in hydrogeologic basins consider balancing of TDS, and independent balancing of individual ions, such as chloride and sulfate. Individual ion calculations must be consistent with cation-anion balance relative to TDS:
(1)For the purposes of discussion, consider the hypothetical case where we will assume that TDS consists of single ion endmembers, chloride (anion) and sodium (cation). In this hypothetical case we will also assume TDS are equal to 500mg/L. What is the maximum chloride and sodium concentration that can occur in this hypothetical example? To determine the maximum concentration we will formulate two sets of equations. In the first equation, the addition of the mass concentration of sodium (X) and mass concentration of chloride (Y) is equal to 500mg/L (TDS):
(2)
Now considering the cation/anion balance, the difference between total cations in meq/L and total anions in meq/L must equal 0, following equation 1. Converting sodium to meq/L and chloride to meq/L by multiplying their mass values by conversion factors:
Where 0.04350 and 0.02921 are conversion factors for changing values from mg/L to meq/L.8 Using equation 1, under the assumption that Na and Cl are the only ions combining to make bulk TDS
(3)
Solving equations 2 and 3 algebraically:
X=200.8=Na (mg/L),
Y=299.2=Cl (mg/L)
In this hypothetical scenario, where the only constituents contributing to TDS are the ions of sodium and chloride, the maximum possible concentration of chloride is about 299mg/L. This must be balanced by a mass concentration of sodium of about 201mg/L. Other major cations (Mg, Ca, K) and major anions (SO4, HCO3) are always present, along with dissolved silica. The calculation above serves to illustrate the mass concentration limits of individual ions, relative to TDS, that are possible in salt balances.19 In reality the concentration of chloride in groundwater in this case would be far less than 299mg/L due to the presence of other anions (and cations). On the point made earlier about the mass of chloride equaling 80% of the total mass of TDS; this has been demonstrated to be an impossible scenario.
TDS may be computed in multiple ways. It is necessary to understand which method(s) of calculation of TDS has been used in historical data sets. This will ensure that an analyst is able to standardize calculation of TDS in time series analysis. There are three common ways to calculate TDS.20 The oldest method (Method 1) is by evaporation of water sample to a residue. In this method, which has been commonly used for over 120 years, a specified amount of filtered water is weighed in an analytical balance and evaporated to a residue. The soluble residue (TDS) left behind is then weighed in an aluminum foil cup. During evaporation to a residue any HCO3 (bicarbonate) present in the water sample is converted to CO3 (carbonate)+CO2+H2O, with 50.8% of the HCO3 mass driven off as CO2 and H2O vapor. When all of the water is evaporated, 49.2% of the HCO3 in solution remains as CO3 solid residue. About half of the original mass of bicarbonate is lost as carbon dioxide and water vapor. Most groundwater samples contain mostly bicarbonate as the primary carbonate species, with little to no CO3. Therefore, the method produces error in the TDS calculation because bicarbonate is cut in half. The error becomes particularly significant when bicarbonate is the most dominant anion mass in the groundwater sample. With the development of automated analytical instruments in the 1970s and 1980s, (e.g., IC, AA, ICP-OES, ICP-MS) it became possible to determine individual ions and non-ionic constituents quickly and reliably. With major constituents available it is possible to estimate TDS by summation of ions and inert elements. This is the primary method for calculating TDS today. One method used by oil field labs and many commercial labs sums major cations, anions and silica; all measured in mg/L:
(Method 2)
Where the major cations are Mg, Ca, K, and Na and the major anions are SO4, HCO3, Cl, and possibly CO3 and NO3 if concentrations of carbonate and nitrate are significant. A third method, which is preferred by the USGS, EPA, and many state agencies, TDS is calculated as follows:
(Method 3)
Method 3 calculates reduction of bicarbonate concentration artificially as done when TDS is calculated by evaporation to a residue (Method 1). Method 3 is used as the calculation of choice by many entities so historical comparisons can be made to TDS calculated in earlier decades by evaporation to a residue (Method 1). Evaporation to a residue was the most widely used method from the late 1800s to the 1960s, and a significant historical data base exists for surface water and groundwater collected and analyzed for TDS by Method 1. Analysts are able to make confident interpretations of times series changes in TDS that were calculated for decades by evaporation to a residue and more recently by summation of ions and silica, balanced by calculated reduction of bicarbonate.20 Time series variations in TDS must be evaluated based on systemic hydrochemical changes in a groundwater basin. Method 2 is the most accurate of the three methods, but because bicarbonate is not reduced to carbonate in the calculation, it does not correspond to historical data sets analyzed by evaporation to a residue. Because all methods have been used by various analysts to perform TDS calculations, it is important to determine what methods were used so that any change in method may be reconciled in empirical and calculated data bases. We reviewed the methodology here so an analyst can consider how to reconcile historic data sets when methods of calculation of TDS may have changed in time.
The salt balance of the soil water zone of a groundwater basin includes multiple components, including inputs and outputs.1 The inputs include salt in natural precipitation falling upon the area, salt in the irrigation water used in an area, residual salts in the soil, and salt dissolved into solution from soil minerals. The outputs include salt in applied fertilizers or soil amendments, salt taken up by or retained on the plant and removed, salt chemically precipitated in the soil, and salt in the drainage water from the area. These inputs and outputs operate in the groundwater zone and in the soil water zone. The soil water zone (e.g., vadose zone) is often the main source of salt loading source to groundwater. Outputs of salts from groundwater include salt mass lost from pumping, river leakage, evapotranspiration, underflow, and small amounts of salts lost by precipitation as mineral cements. Groundwater formed in rocks and sediments of different origins have dissolved ions reflecting the dissolution and weathering products of specific minerals in igneous rocks, sedimentary rocks, metamorphic rocks, and basin fill alluvium. Groundwaters recharged and flowing through sedimentary and metamorphic rocks generally have much higher dissolved solids content than groundwater contained in igneous rocks. Igneous rocks include volcanic and intrusive rocks of magmatic origin that are dominated by minerals rich in silica. Common minerals in igneous rocks include silica rich rocks such as quartz, feldspars, pyroxene, amphibole, and olivine. Many such minerals have generally low solubility compared to carbonates and evaporates.4 This fact accounts for the dilute salt concentrations found in most groundwaters in igneous rocks. In basin fill, the dissolved solids are dependent on the rock fragments and minerals present in the fill material. The surface area of sediment grains in contact with groundwater is greater than the surface area in contact with rock matrix in igneous and metamorphic rocks, leading to more enhanced potential for water-rock interactions.
The presence of soluble marine rocks and detritus in groundwater basins often creates high salt loading to soils and aquifers due to in situ mineral dissolution. Where sediments in aquifers are derived from erosion of igneous highlands loading factors due to dissolution of mineral mass are usually low. Geologic materials account for considerable contrasts in groundwater salinity. Based on the analysis of the geological inputs, these natural sources of salinity, particularly where sedimentary rocks are present, must be included in Salt Balance Analysis. Where recycled water and tap water are the recharge sources, the soluble salts of sulfate and chloride are not inhibited via dissolution of soluble minerals. Minerals such as gypsum and halite are quite soluble and dissolution processes proceed rapidly when these minerals are present. To improve estimates in the face of uncertainty, empirical “field” studies should be carried out to determine what are the anticipated loading rates of salts and water due to irrigation with recycled water. Without additional studies, the evaluations must consider average as well as high/low loading scenarios, as performed in this investigation. Uncertainty must factor in how much difference is represented in the high/low calculations. If the amount of recycled water used produces limited changes to the salt loading in a basin, even in “high percentage” loading scenarios, then the impact of loading of salts due to recycled water use might not be evaluated further. If the “high-low percentage” loading scenarios produce significant differences, then additional work should focus on improving estimates in a groundwater basin. Finally, methods regarding cation-anion balance and calculation of TDS must follow strict guidelines to make sure time series comparisons of multiple data sets are consistent and appropriate ion values are used to be consistent with TDS estimates.
None.
Author declares there is no conflict of interest.

©2018 Hibbs. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 World Water Day is celebrated globally on March 22nd. Water is the most essential source for sustaining
life, and it plays an important role in the well-being of every human being. From the International Journal of Hydrology, we wish to convey
to everyone the vital importance of water as a fundamental part of human life. To support this cause, we invites researchers to contribute
articles that highlight the importance of Water. To encourage participation, IJH is offering a 30% discount on all submissions received on
or before March 22nd.
World Water Day is celebrated globally on March 22nd. Water is the most essential source for sustaining
life, and it plays an important role in the well-being of every human being. From the International Journal of Hydrology, we wish to convey
to everyone the vital importance of water as a fundamental part of human life. To support this cause, we invites researchers to contribute
articles that highlight the importance of Water. To encourage participation, IJH is offering a 30% discount on all submissions received on
or before March 22nd.