International Journal of
eISSN: 2576-4454


Research Article Volume 2 Issue 1
Department of Environmental Science, Federal Urdu University, Pakistan
Correspondence: Waqar Ahmad, Department of Environmental Science, Federal Urdu University, Pakistan
Received: November 09, 2017 | Published: January 4, 2018
Citation: Zafar MU, Ahmad W. Water quality assessment and apportionment of northern pakistan using multivariate statistical techniques – a case study. Int J Hydro. 2018;2(1):1-7. DOI: 10.15406/ijh.2018.02.00040
The present study examines the physico-chemical characteristics in Gilgit and Hunza rivers, northern Pakistan. Twenty-nine water samples from different locations of Gilgit and Hunza rivers were collected during July 2012. The analytical results of the samples point out high concentration of bicarbonates. Large variations in the level of various parameters including chloride, total alkalinity, total hardness, sulphates and nitrates were observed. Multivariate statistical techniques were employed to interpret the data and results of physico-chemical properties showed that values of all parameters were in accordance with the permissible limits proposed by World Health Organization guidelines. The high values of total alkalinity showed that surface water of the study area is of bicarbonate type.
Keywords: physico-chemical characteristics, gilgit and hunza river, multivariate statistical analysis
The Indus is one of the largest river of Pakistan with drainage basin area (9,70,000 km2). It is nourished by glaciers and snows of the Himalayas, the Karakorum and the HinduKush that originate in the Indian State of Jammu and Kashmir and the Northern Areas of Pakistan. The parts of the HinduKush and the Karakorum ranges in the northern territory of Pakistan are drained by Gilgit River (current study area) which is bordered with Afghanistan and China in the North. The Hunza River basin is also a part of the present study area, actually the sub basin of the Gilgit River but owing to its substantial size and significance, it is considered as a separate basin. Like Gilgit River, Hunza River also drains the Karakorum Mountains consisting of a large glaciated area situated in the North. As far as water analysis is concerned, little work has been carried out in these areas. Mercury in Pan Amalgamation was found in high concentration in 37 river samples collected from 24 different sites of Gilgit and Hunza.1 Physico-chemical properties along with some heavy metals (arsenic, chromium, copper, mercury and lead) of Handrap Lake and Nullah in Ghizer district were tested and no trace metals were found (World Wildlife Fund). As Northern areas of Pakistan are mountainous rural regions with a population of 900,000 living in villages typically encompassing 50-200 households.2 In this region, the main source of water supply is from melting snows. It comes through channels (river lets) and small streams to the mouth of villages and is considered as the only supply of water for the villages as there are no wells and hand pumps which can substitute for this water. The water from melting snows run down the hills collecting various materials on its way and converted to turbulent mountainous streams.3 The utilization of such water for domestic purpose may cause harmful diseases. So here we propose a study, where water samples were selected from 29 locations of Gilgit and Hunza Rivers (Figure 1) to check the quality of water from study areas. The following objectives were covered.
Research objectives:
Sampling and on site evaluation
Twenty nine locations were chosen from Gilgit and Hunza Rivers for the estimation of physico-chemical parameters. The first 12 locations were from Gilgit River and the last 17 locations were selected from Hunza River (Table 1). Samples were collected in the month of July 2012. The collection was carried out in such a way that samples did not get contaminated with other substances. At each site, surface water was collected and kept in polythene plastic bottles formerly washed in 10% nitric acid for 24 hours and rinsed with distilled water. 500 ml water was collected in each bottle and six parameters were noted at the site with the assistance of Sension 156 HACH potable multi-parameter, USA. The parameters were temperature (oC), pH, electrical conductivity (µS/cm), total dissolved solids (mg/L), salinity (%) and dissolved oxygen (mg/L). Two-three drops of nitric acid were introduced in the bottles so that chemical characteristics of the water could not be deteriorated.
S. no. |
Locations |
Elevation in meters |
Co-ordinates |
1 |
Napura Baseen |
1683 |
35o50N, 74o15E |
2 |
Napura Baseen (spring) |
1700 |
35o50N, 74o15E |
3 |
Kargah |
1674 |
35o50N, 74o15E |
4 |
Gilgit city |
1574 |
35o54N, 74o21E |
5 |
Gilgit tap water |
1574 |
35o54N, 74o21E |
6 |
Jutial |
1748 |
35o53N, 74o20E |
7 |
Nomal |
2507 |
36o08N, 74o12E |
8 |
Nalter (spring) |
2968 |
36o07N, 74o10E |
9 |
Nalter (Lake) |
2968 |
36o07N, 74o10E |
10 |
Danyore |
1580 |
36o08N, 74o51E |
11 |
JuglotGah Nala |
1610 |
36o09N, 74o51E |
12 |
HaramoshNala |
1600 |
35o07N, 74o08E |
13 |
Aliabad Nala |
1700 |
36o09N, 74o52E |
14 |
Aliabad tapwater |
1700 |
36o09N, 74o52E |
15 |
Atabad |
2400 |
36o20N, 74o52E |
16 |
Gulmit |
2412 |
36o20N, 74o52E |
17 |
Hussaini |
2433 |
36o20N, 74o52E |
18 |
Ghalapur Nala |
2500 |
36o36N, 74o51E |
19 |
Khyber Nala |
2678 |
36o34N, 74o48E |
20 |
Passu |
2700 |
36o46N, 74o90E |
21 |
Gulkin Nala |
2403 |
36o24N, 74o52E |
22 |
Batura Glacier |
2540 |
36o30N, 74o52E |
23 |
Batura Lake |
2540 |
36o30N, 74o52E |
24 |
Shimshal River |
2850 |
36o20N, 75o01E |
25 |
Shimshal |
2850 |
36o20N, 75o01E |
26 |
Morkhun |
2780 |
36o40N, 74o52E |
27 |
Boiber tributary |
3075 |
36o40N, 74o52E |
28 |
BoiberNala |
3075 |
36o40N, 74o52E |
29 |
Sost River |
3075 |
36o41N, 74o52E |
Table 1 Nearest town, elevation and map location of water collection of Gilgit and Hunza valleys
Laboratory and statistical analyses
In the laboratory, chemical analyses were carried out to find chloride, total hardness, total alkalinity, nitrate and sulphate. Chloride, total hardness, and total alkalinity were detected by titration method while nitrate and sulphate were evaluated by spectrophotometer; detail of the methods is given in Table 2. For statistical analysis, “Minitab” version 11.12 was used. A Pearson Correlation matrix among all parameters was created to check whether the concentration of one parameter affect the concentration of another. Multivariate techniques (cluster analysis and principal component analysis) were applied to the datasets by normalizing the variables.
Serial no. |
Variables |
Abbreviation |
Analytical method |
Units |
|
physical |
parameters |
|
|
1 |
Temperature |
Temp |
Sension HACH,156 |
oC |
2 |
pH |
pH |
Sension HACH,156 |
No |
3 |
Electrical Conductivity |
EC |
Sension HACH,156 |
µS cm-1 |
4 |
Total Dissolved Solids |
TDS |
Sension HACH,156 |
mg L-1 |
5 |
Salinity |
Sal |
Sension HACH,156 |
% |
Chemical |
Parameters |
|||
6 |
Dissolved Oxygen |
DO |
Sension HACH,156 |
mg L-1 |
7 |
Chloride |
Cl-1 |
Titration (Silver Nitrate) |
mg L-1 |
8 |
Total Hardness |
Ca+Mg |
Titration (EDTA) |
mg L-1 |
9 |
Total Alkalinity |
CO3+HCO3 |
Titration (H2SO4) |
mg L-1 |
10 |
Sulphate |
SO4 |
Spectrophotometer |
mg L-1 |
11 |
Nitrate |
NO3 |
Spectrophotometer |
mg L-1 |
Table 2 Analysis parameters and their analytical procedures
The procedures followed were those described by APHA.18
The values of twenty nine water samples with eleven parameters are presented in Table 3. World Health Organization limits for safe drinking water are shown at the bottom of Table 3. We took averages of all parameters from two rivers and then compared these averages. Temperature, pH, DO, salinity, chloride and nitrate had similar values. The other parameters TDS, conductivity, total alkalinity, total hardness and sulphate showed dissimilarities in results. The higher values were obtained from Hunza River explaining Hunza River in part because of the Boiber tributary which exceeded the WHO (1993) approved thresholds of pH, conductivity, total alkalinity and total hardness as was the case with Boiber Nala tributary. The values of all parameters satisfied the corresponding thresholds (except in a few cases), whereas total alkalinity was found to be high. This means that all samples have high values of alkalinity. Total Dissolved Solids, D.O, salinity, chloride, sulphate and nitrate met the acceptable limits designed by WHO (1993).
S.no |
Locations |
Temperature |
ph |
D.o |
T.d.s |
Conductivity |
Salinity |
Chloride |
Total Alkalinity |
Total Hardness |
Sulphate |
Nitrate |
1 |
Baseenpur |
13.4 |
8.54 |
0.98 |
23 |
40.2 |
0 |
14 |
420 |
40 |
10 |
11.1 |
2 |
Baseenpur (Spring) |
12.31 |
7.57 |
0.91 |
22.3 |
35.7 |
0.1 |
10 |
460 |
32 |
10 |
27.4 |
3 |
Kargah |
13 |
7.6 |
1.8 |
24.3 |
38.2 |
0.1 |
10 |
700 |
32 |
11 |
10.9 |
4 |
Gilgit City |
27.6 |
8.1 |
1.7 |
31 |
69.5 |
0 |
10 |
480 |
48 |
18 |
37.4 |
5 |
Gilgit tap water |
25.2 |
7 |
1.65 |
35 |
83.6 |
0.1 |
9.93 |
1200 |
40 |
11 |
32.9 |
6 |
Jutial |
12.1 |
7.48 |
1.89 |
27.6 |
43.6 |
0.1 |
12 |
560 |
28 |
19 |
12.4 |
7 |
Nomal |
12.9 |
8.65 |
1.29 |
110 |
68.6 |
0.1 |
8 |
1020 |
120 |
22 |
5 |
8 |
Nalter (Spring) |
7.4 |
8.2 |
2.2 |
24.8 |
38.5 |
0.1 |
10 |
360 |
132 |
11 |
36.7 |
9 |
Nalter lake |
9.4 |
7 |
0.49 |
61.4 |
145.7 |
0 |
7.94 |
960 |
80 |
21 |
9.5 |
10 |
Danyore |
16.5 |
7.5 |
1.73 |
86.7 |
204 |
0 |
9.93 |
1020 |
100 |
37 |
21 |
11 |
JuglotGahNala |
12.4 |
7.77 |
1.5 |
211 |
505 |
0.1 |
12 |
860 |
80 |
23 |
9.7 |
12 |
HaramoshNala |
14.2 |
7.4 |
1.74 |
102.2 |
240 |
0.1 |
11.91 |
900 |
140 |
43 |
26.3 |
13 |
Aliabad Nala |
12.3 |
7.1 |
1.63 |
36.5 |
86.1 |
0 |
9.93 |
840 |
40 |
18 |
22.5 |
14 |
Aliabad Tawafer |
13.2 |
7.42 |
1.48 |
47.7 |
115.4 |
0 |
12 |
920 |
220 |
119 |
18.5 |
15 |
Atabad |
10.2 |
7.4 |
2.01 |
69.7 |
164 |
0 |
7.94 |
700 |
100 |
21 |
29.4 |
16 |
Gulmit |
12.1 |
7.86 |
1.47 |
85.7 |
20.3 |
0 |
12 |
980 |
72 |
32 |
18.5 |
17 |
Hussaini |
10.2 |
7.75 |
1.41 |
71.21 |
168.9 |
0 |
6 |
820 |
100 |
24 |
16.4 |
18 |
GhalapurNala |
11.8 |
8.49 |
1.36 |
166.9 |
70.4 |
0 |
12 |
700 |
116 |
9 |
13.7 |
19 |
Khyber Nala |
16.7 |
8.54 |
1.85 |
171.8 |
316 |
0.1 |
12 |
740 |
172 |
78 |
10.8 |
20 |
Passu |
11.3 |
7.2 |
1.7 |
52.6 |
124.4 |
0 |
11.9 |
880 |
80 |
34 |
33.2 |
21 |
GulkinNala |
10.8 |
8.37 |
2.07 |
19.3 |
36.3 |
0 |
12 |
540 |
36 |
9 |
17.6 |
22 |
Batura Glacier |
11.1 |
8.55 |
2.02 |
52.3 |
96.2 |
0 |
12 |
600 |
48 |
19 |
33.8 |
23 |
Batura Lake |
11.1 |
8.3 |
1.98 |
119.9 |
219 |
0.1 |
10 |
1000 |
168 |
46 |
31.5 |
24 |
Shimshal River |
10.9 |
7 |
2.01 |
78.3 |
184.8 |
0 |
14.89 |
800 |
120 |
48 |
36.5 |
25 |
Shimshal |
11 |
7.2 |
1.92 |
181 |
423 |
0.1 |
9.93 |
860 |
240 |
91 |
39.9 |
26 |
Morkhun |
13.3 |
8.75 |
1.26 |
182 |
426 |
0.1 |
8 |
840 |
136 |
79 |
36 |
27 |
BoiberTributery |
14.5 |
8.55 |
1.46 |
277 |
513 |
0.3 |
10 |
820 |
176 |
86 |
12.1 |
28 |
BoiberNala |
13.2 |
8.73 |
1.7 |
155 |
290 |
0.1 |
8 |
560 |
196 |
60 |
42.7 |
29 |
Sost River |
13.5 |
8.26 |
1.7 |
109.5 |
187.5 |
0.1 |
14 |
900 |
140 |
50 |
12.4 |
|
WHO (1993) |
No guideline |
6.5-8.5 |
No guideline |
500 mg/L |
400 µS/cm |
No guideline |
250 mg/L |
250 mg/L |
150 mg/L |
500 mg/L |
50 mg/L |
Table 3 Values of all sampling sites with eleven parameters from Gilgit and Hunza Rivers
The Pearson correlation matrix of all parameters
Correlations of all parameters from 29 samples of Gilgit and Hunza Rivers are shown in Table 4. Eleven parameters from 29 locations from two rivers were analyzed for correlation. The significance level at degrees of freedom 27 is checked using r Table in the following order.
Temperature did not illustrate any significant correlation with all parameters. It was negatively correlated with pH, D.O, TDS, conductivity and total hardness and positively correlated with salinity, chloride, total alkalinity, sulphate and nitrate. pH was found to be significantly correlated with total dissolved solids and total alkalinity at (p<0.05) and positively correlated with all parameters except chloride and nitrate. No significant correlation was found between dissolved oxygen and all other parameters. It was positively correlated with three parameters and negatively correlated with the other parameters. TDS showed significant correlations with four parameters i.e. conductivity, salinity, total hardness and sulphate at (p<0.001). Conductivity also expressed strong significant positive correlations with salinity, hardness and sulphate and weak positive correlation with total alkalinity. Positive significant correlations were observed among salinity, total hardness and sulphate at (p<0.05) level and chloride did not show any significant correlation with any other parameter. Total alkalinity and total hardness indicated significant positive correlation with sulphate at (p<0.05) and (p<0.001) respectively. There was no significant correlation between sulphate and nitrate. This showed that total hardness and sulphate are the two parameters which are highly correlated with most of the other parameters. The characteristics of three groups derived from agglomerative cluster analysis are presented (Table 5 & Figure 2). Temperature was similar in all clusters. The pH of the water was slightly more alkaline for cluster I and III. Dissolved oxygen was almost similar in all groups whereas total dissolved solids were remarkably higher in group III. Conductivity of the samples showed greater values for groups II and III. Salinity was found to be higher in cluster III while chloride was little bit higher in group I. Total alkalinity was found to be low in cluster I as compared to the other two clusters. Total hardness of group III was higher than the other two groups. Sulphate and nitrate showed greater values for group III. Principal Component analysis (PCA) was applied on normalized data sets (11 variables) separately for 29 locations (Figure 3) to find similarities or dissimilarities among variables. PCA of the data sets produced first five PCs with eigen values > 1 explaining 80.8 percent of total variance with respect to water quality data. Eigen value measures the significance of the factor and values greater than one are considered as significant contribution to the analysis.4,5 Six parameters (temperature, pH, D.O, salinity, chloride and nitrate) formed a close association with sulphate. Hardness and TDS centroids occurred close together but were distinct from the previous 7 parameters. The conductivity and alkalinity centroids were found to be located quite apart from the other parameters in ordination space, indicating they have the least correlation with the other parameters. The scree plot (Figure 4) was used to explain the number of PCs to be retained in order to understand the fundamental data structure.6 The scree plot of the present study showed that first five PCs have eigen values greater than one (Figure 4), together the first four components explains 70.4% of the total variance inherent in the data set. Among three PCs, PC1 explaining 33% of total variance has weak positive loadings on TDS, conductivity, salinity, total hardness and sulphate. PC2 (14.5% of total variance) has moderate positive loadings on D.O and nitrate which may indicate that increased concentration of nitrate will presumably increase the consumption of dissolved oxygen7 PC3 with 13.4% of total variance caused moderate positive loading over pH and alkalinity. Overall analysis of PCA shows that variations of water quality are related to dissolved solids and salts (e.g., sulphate, nitrate, total alkalinity and total hardness) which may change the pH and dissolved oxygen concentration of water of the study area.
|
Temperature |
ph |
D.o |
Tds |
Conductivity |
Salinity |
Chloride |
Total Alkalinity |
Total Hardness |
Sulphate |
ph |
-0.006 |
|||||||||
D.o |
-0.067 |
0.2 |
||||||||
Tds |
-0.039 |
0.379 |
0.216 |
|||||||
Conductivity |
-0.01 |
0.149 |
-0.134 |
0.877 |
||||||
Salinity |
0.088 |
0.288 |
-0.165 |
0.592 |
0.561 |
|||||
Chloride |
0.014 |
-0.023 |
0.131 |
-0.093 |
-0.137 |
-0.125 |
||||
Total Alkalinity |
0.155 |
-0.358 |
-0.071 |
0.289 |
0.273 |
0.067 |
-0.133 |
|||
Total Hardness |
-0.166 |
0.189 |
0.039 |
0.638 |
0.603 |
0.354 |
-0.099 |
0.253 |
||
Sulphate |
0.001 |
0.083 |
-0.18 |
0.565 |
0.631 |
0.349 |
0.032 |
0.301 |
0.843 |
|
Nitrate |
0.126 |
-0.122 |
-0.152 |
-0.089 |
0.084 |
-0.09 |
-0.131 |
-0.156 |
0.205 |
0.142 |
Table 4 Correlation matrix among all parameters
Water quality variables |
Cluster I (1,2,4,8,3,18,6,21,22) |
Cluster II (5,7,16,9,10,23,12,29,14,13,20,15,17,24) |
Cluster III (11,27,25,26,19,28) |
Temperature |
13.28 |
13.07 |
13.51 |
ph |
8.1 |
7.56 |
8.25 |
D.o |
1.66 |
1.59 |
1.61 |
T.d.s |
43.5 |
76.17 |
196.3 |
Conductivity |
52.07 |
143.73 |
412.16 |
Salinity |
0.04 |
0.035 |
0.13 |
Chloride |
11.33 |
10.45 |
9.98 |
T. Alkalinity |
535.56 |
924.2 |
780 |
T. Hardness |
56.89 |
108.5 |
166.66 |
Sulphate |
12.89 |
37.5 |
69.5 |
Nitrate |
22.33 |
22.4 |
25.2 |
Table 5 Characteristics of three groups derived from Ward’s clustering of the water quality variables of the samples collected from 29 locations
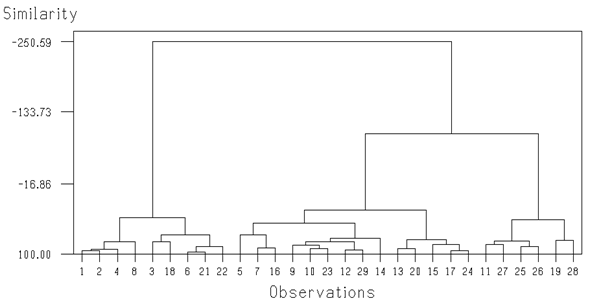
The present study is the first comprehensive study in which we investigated water resources (river, stream, lakes and nullahs) of Gilgit and Hunza valleys. Mean conductivity was satisfactory among all samples except that water from Juglot Gah Nala exhibited high conductivity. This sample also had a high amount of TDS, perhaps this might be due to the presence of minerals in the water originating from solid rocks and glaciers. Of all parameters, only total alkalinity crossed the tolerable threshold of drinking water. The rocks of these valleys contain high amounts of carbonate and bicarbonate which mix with the water passing through it, causing the high water alkalinity. Correlation analysis results explained that there are specific relationship pattern among pH, TDS, conductivity, total hardness and sulphate and these results are also confirmed by principal component analysis. Correlation analysis results showed the direct significant positive relationship (P<0.001) between electrical conductivity with total hardness and sulphate. It means that total hardness and sulphate drive the conductivity of surface water of Rivers as reported in ground water from urban areas of Karachi.8 The cluster analysis of the overall data set showed three major groups while the discriminating variable for the groups were six variables (temperature, pH, D.O, salinity, chloride and nitrate) which showed homogeneity within groups but heterogeneity between the three groups. TDS and hardness also exhibited considerable differences between the groups. Likewise, vast differences in the mean values were observed between the clusters for conductivity and total alkalinity. Cluster analysis highlights that at present, most of the samples collected from Hunza River have high values of TDS, conductivity, total alkalinity and hardness which may indicate the high concentration of salts in Hunza River as compared to Gilgit River. In the present study, we found the lowest temperature (7.4oC and 9.4oC) from Nalter site (lake and spring respectively). The low temperature of the water is due to the fact that the water which we collected from Nalter, was coming from glaciers. Similar temperature (8oC) was also observed by Islamuddin (2010) who worked over Nalter Lake. Water temperature from Nomal valley, located at lower height (2507 m) than Nalter valley (2968 m) was 13oC in current study whereas9 described the temperature of the same valley about 25oC due to the difference in collection seasons. All the physico-chemical properties from the present study and the Islamuddin10 study are concurrent with each other and are in accordance with the limits of WHO (1993) except total alkalinity (Carbonate and bicarbonate) were relatively higher in both studies. Our physico-chemical characteristics also match the findings of Jhelum River, District Muzzafarabad Azad Kashmir study11 and with that of some studies of lower Indus Basin12−14 but total alkalinity was lower in their studies as compared to current studies. The concentrations of all chemical parameters were found within permissible limits in samples with the exception of total alkalinity in the samples. The alkaline water has a bitter taste and slippery feel. High alkaline water like in the current study can cause drying of skin. Alkalinity is important for fish and aquatic life because it acts as a buffer against rapid pH changes.15,16 High alkalinity is also important in agricultural activity. Use of high alkaline water affects plant growth because excessive salts raise osmotic pressure in soil solution and reduce water availability. This high alkalinity results in lower leaf-area index.17 Finally it is concluded that physico-chemical properties of surface water of study areas are within drinking permissible limits of WHO (1993) but the water is considered as bicarbonate type. It is still important to explore the bacterial contamination of the study area but it is not a current study objective. However Nano-filtration techniques should be installed at the mouth of water supply to reduce the total alkalinity of Gilgit and Hunza Rivers.18
None.
Authors declare there is no conflict of interest in publishing the article.

©2018 Zafar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 World Water Day is celebrated globally on March 22nd. Water is the most essential source for sustaining
life, and it plays an important role in the well-being of every human being. From the International Journal of Hydrology, we wish to convey
to everyone the vital importance of water as a fundamental part of human life. To support this cause, we invites researchers to contribute
articles that highlight the importance of Water. To encourage participation, IJH is offering a 30% discount on all submissions received on
or before March 22nd.
World Water Day is celebrated globally on March 22nd. Water is the most essential source for sustaining
life, and it plays an important role in the well-being of every human being. From the International Journal of Hydrology, we wish to convey
to everyone the vital importance of water as a fundamental part of human life. To support this cause, we invites researchers to contribute
articles that highlight the importance of Water. To encourage participation, IJH is offering a 30% discount on all submissions received on
or before March 22nd.