International Journal of
eISSN: 2573-2838


Research Article Volume 1 Issue 1
1Laboratories for Nanoscience and Nanotechnology Research, Sri Sathya Sai Institute of Higher Learning, India
2Department of Physics and Astronomy, Clemson University, USA
Correspondence: Venkataramaniah Kamisetti, Laboratories for Nano science and Nanotechnology Research, Sri Sathya Sai Institute of Higher Learning, Prasanthinilayam, India
Received: September 30, 2016 | Published: November 4, 2016
Citation: Kamisetti V, Mulpur P, Kurdekar A, et al. Surface plasmon coupled emission enabled silver-C60 nano-biosensors for sensitive detection of tuberculosis. Int J Biosen Bioelectron. 2016;1(1):18–22. DOI: 10.15406/ijbsbe.2016.01.00005
The current global scenario for diagnosing the fatal tuberculosis (TB) disease includes techniques like chest X-ray that show cavitary lesions indicating the presence of an active TB infection. This is often further substantiated with a confirmatory diagnosis involving the fluorescence microscopy aided positive identification of the causative organism; Mycobacterium tuberculosis (Mtb) in the blood or sputum of patients. However, expensive infrastructure in the form of X-ray and cell-culture/fluorescence microscopy facilities may not be present in resource limited settings (RLS) of the under-developed and developing nations where TB is highly prevalent. Furthermore; the standard microscopy based detection of TB bacteria involving fluorescence is limited by the isotropic nature of the fluorescence phenomenon itself, which is innately associated with poor signal collection efficiency and low signal-to-noise ratios. These limitations that hinder the early and positive identification of the TB disease condition may result in even the death of the patient. Therefore, in addressing this crucial need for developing efficient sensing strategies; we present the novel Surface Plasmon Coupled Emission (SPCE) sensing platform that enables the generation of highly amplified fluorescence signals with excellent signal collection efficiency, from low sample volumes; as a promising tool for enabling the highly sensitive and rapid diagnosis of tuberculosis. In this regard, we have employed Ag-C60 thin-film substrates, which we have previously reported as low-cost and highly sensitive fluorescence sensor platforms, along with the well-established acid-fast fluorescence staining protocol that offers specificity to detection of Mtb. This synergistic approach allowed us to perform the early, sensitive and specific diagnosis of TB in an economically viable manner.
Tuberculosis or TB (tubercle bacillus), which is also known as phthisis, phthisis pulmonalis, or consumption; is a widespread and highly infectious disease that is usually caused by Mycobacterium tuberculosis, which is a pathogenic bacteria belonging to the mycobacterium genus.1 Tuberculosis typically attacks the lungs, but can also affect other parts of the body. Tuberculosis is considered to be highly infectious in nature. Most infections do not show any symptoms (asymptomatic) and are hence known as cases of latent tuberculosis. From statistical analysis it has been determined that, about one in ten latent infections eventually progresses to the active disease state, which if left untreated, kills more than 50% of those infected.
Considering the threat TB poses, the diagnosis of TB is usually performed by performing chest X-rays of infected patients and microscopy analysis of blood/sputum samples. Although a chest X-ray showing cavitary lesions in the lung of infected patients is suggestive of the presence of TB, there are other lung infections that may exhibit similar X-ray profiles. In this regard, the definitive diagnosis of TB can be confirmed on the positive microscopic observation of Mycobacterium tuberculosis (Mtb) cells.2 Mtb cells exhibit the typical rod-shaped morphology, characteristic to bacilli. In this procedure, clinical samples from suspect patients; such as sputum, pus or tissue biopsy samples are first collected. The bacterial cells are isolated and cultured after which, they are suitably stained and usually observed under a fluorescence microscope. The positive microscopic identification of the rod-shaped Mtb cells from the clinical patient samples forms a definite diagnosis of activeTBis considered as the ‘Gold Standard’ among all the other currently available diagnostic protocols.
Although, it is the ‘Gold Standard’ diagnostic technique for ascertaining the presence of TB infection, microscopy requires the culturing of TB cells as a prerequisite. This is a bottleneck limitation because, TB divides at an extremely slow rate of 16-20 hours per division, which is drastically slower compared to other bacteria that usually divide within an hour. Therefore, the culturing of Mtb cells before performing microscopy is an extremely long process that can take up to anywhere between 2-6 weeks, for samples collected from sputum or blood. Considering the dangerous progression of TB disease, delayed diagnosis can prove to be fatal. Furthermore, the entire process of culturing and performing fluorescence microscopy of Mtb cells is a highly time consuming and tedious process. Finally; both the X-ray scanning technique and the cell-culture/fluorescence microscopy based diagnostic methods are expensive, where the necessary sophisticated infrastructure may not be available in the resource limited settings (RLS) of certain under-developed and developing nations of the world where TB is prevalent. Pertaining to fluorescence microscopy, it is noteworthy that fluorescence is an isotropic phenomenon wherein the signal collection efficiency is <1% in traditional optical configurations. In addition, the fluorescence signal intensity arising from low sample concentrations would be very difficult to discern and may also be also be associated with auto-fluorescence and background fluorescence which detrimentally lower the signal-to-noise ratios.
In our work, we have addressed these pertinent issues of specificity and sensitivity to achieve the early and ultrasensitive detection of TB in the following manner:
Specificity
We have been able to specifically identify Mtb by exploiting the acid-fast nature of Mtb. Acid-fastness is a property of a bacterium owing to the presence of a hydrophobic waxy lipid known as mycolic acid within their thick cell walls; that is resistant to decolorization by acids and/or alcohols during the staining procedure. The acid-fast staining protocol takes advantage of this acid-fastness of mycobacteria (Mtb); and helps in their specific detection amidst other non-acid-fast bacteria (thin cell walls with no mycolic acid).3 Therefore, only stains, dyes or other fluorophores that are present along with a hydrophobic system can penetrate the cell walls of acid-fast organisms. The rhodamine b (RhB) dye used in the present study in combination with glycerol and phenol is a well-known and good acid fast dye that preferentially allows the identification of only acid-fast microorganisms.
Sensitivity
As mentioned before, even though fluorescence based sensing is a very good procedure, the sensitivity is fundamentally limited by the isotropic nature of the phenomenon. In addressing this crucial need for developing efficient sensing strategies; we present the Surface Plasmon Coupled Emission (SPCE) sensing platform as a promising tool for enabling the highly sensitive, specific and rapid diagnosis of tuberculosis. In SPCE, the emission from excited fluorophores (that are in the vicinity of thin metal films) couples to the plasmon modes of the underlying metal layer that results in the generation of plasmon coupled fluorescence signals, which are highly directional in nature and are characterized with very high signal intensities.4 While traditional SPCE platforms allowed the detection of analytes with high sensitivity; in our previous studies, we have reported the fabrication and demonstration of low-cost Ag-C60 thin-film substrates as optimal nanobiosensor systems that allowed the ultra-amplification of fluorescence signals, which led to unprecedented ultra-sensitive detection of crucial analytes.5 This enables the usage of SPCE substrates sensing platforms where the fluorescence signals generated in response to a sensing event are visually discernible, whereby the fluorescence microscope infrastructure is no longer necessary. In this manner, making use of the acid-fast procotol and the Ag-C60 SPCE platform that requires a simplistic experimental setup, we report the highly specific and sensitive detection of Mtb in an economically viable manner, which is highly relevant to the current global need for developing smart TB sensors.
Fabrication of GLAG-c60 thin-film architectures
Ag thin-films (~50 nm) were first deposited on pyrex glass microscope slides (75 x 25 mm) via physical vapor deposition to initially form the glass-Ag (GLAG) substrates. Subsequently, about 50 mg of C60 powder was loaded into a tungsten evaporation boat and sublimed onto the 50 nm thick Ag films to form fullerene spacer films of desired thickness (~10 nm). Both Ag and C60 films were deposited in a highly controlled manner at 10-5 Torr.
Culturing, fixing and staining of acid-fast and non-acid-fast bacteria
All the bacterial isolates used in this study are ATCC cultures that were procured from Sunshine Hospitals, Secunderabad, India. The following pure cultures were used: Mycobacterium tuberculosis (Mtb) ATCC 27294, Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923. Bacteria belonging to different genus have varied nutritional requirements for their growth. Therefore, specific and customized growth media are prepared for culturing the different bacteria. In this regard, Löwenstein-Jensen medium (LJ medium)6 is specially used for culturing the acid-fast M. tuberculosis while, the non-acid-fast E. coli and S. aureus are usually grown on a nutrient agar medium.7
Culturing of M. tuberculosis on LJ medium
Although, SPCE based detection may not require bacterial culturing to increase the number of bacteria, we have followed the standard culture protocols for standardization.
Composition of LJ medium:LJ medium consists of coagulated hens’ egg, mineral salt solution, potato flour or potato starch, asparagine, glycerol and Malachite Green dye.
Preparation of LJ medium:We followed the well-established, standard protocols for preparing the LJ medium. For our studies, we purchased the LJ medium from Hi Media. Initially 12 ml of glycerol was added to 600 ml of distilled water. Subsequently, approximately 37.24 g of the LJ media powder was suspended in the prepared solution. The suspension was subjected to constant mixing and heating just until the boiling point, following which, it was sterilized by autoclaving at 121°C, under 15 lbs. pressure for 15 minutes. In this manner, the LJ medium base was prepared. Upon coagulation, the resultant obtained LJ media appeared in a pale bluish green color; and the prepared slants were smooth and opaque.
Inoculation and culturing of M. tuberculosis: After performing the standard sterilizing and decontaminating procedures, the ATCC certified M. tuberculosis parent culture was inoculated into the tubes (with an inoculation loop) containing the prepared LJ medium, in a laminar airflow bio-safety cabinet. The inoculated medium was then incubated and stored in a CO2 atmosphere at 35-37°C, under dark conditions as Malachite Green is photosensitive. During the incubation process that lasts a week, the screw-caps were loosened to ensure CO2 circulation, which is crucial for initiating bacterial growth. After a week, the caps were tightened, to prevent the dehydration of the LJ growth medium. The media in the growth tubes was periodically examined for a period of 8 weeks to monitor the growth and check for any contamination. After this prescribed period, the M. tuberculosis bacteria were identified as brown or buff colored granular colonies.
Culturing of E. coli and S. aureus on nutrient agar medium
The non-acid fast bacteria used in our studies were grown on the standard nutrient agar medium.
Preparation of nutrient agar medium for non-acid-fast bacteria:The nutrient agar media used in our studies was prepared according to the directions provided by Hi Media labs. Peptone and sodium chloride (5 g each), yeast extract and beef extract (1.5 g each), and agar (15 g) were added to 1 L of distilled water. These ingredients were mixed thoroughly under room temperature conditions with pH maintained within a range of 6.4-7.6. Subsequently, the resultant mixture was sterilized by boiling for approximately one minute. After sterilization, the mixture was allowed to naturally cool to around 50°C, upon which it was poured into petri dishes which were immediately covered to avoid contamination. On reaching room temperature the media in the petri dishes were completely solidified due to the presence of agar.
Inoculation and culturing of E. coli and S. aureus innutrient agar medium:The ATCC certified E. coli and S. aureus bacterial cultures are usually stored in glycerol stock solutions, under refrigerated conditions. A sterile inoculation loop was used to isolate the bacterial cultures from the stock, which were then streaked across the solid nutrient agar media in the petri plates. The plates were inverted and incubated overnight at 37°C. Distinct circular colonies were found to have appeared after incubation, which indicated the presence of the non-acid-fast bacteria. The E. coli colonies can be easily differentiated based on their colorless translucent appearance, whereas, the S. aureus colonies appear yellowish and are opaque.
Fixing acid-fast and non-acid-fast bacteria on Ag-C60 SPCE Substrates
On having prepared the sub-cultures of the acid-fast M. tuberculosis and non-acid-fast E. coli and S. aureus bacteria; a sterile inoculation loop was used to transfer approximately equal amounts of the bacterial colonies, into test tubes containing saline solution. The bacterial cultures were handled inside a bio-safety cabinet, under sterile conditions. The saline suspensions of all the three different bacteria were subsequently pipetted onto the GLAG-C60 SPCE substrates. The bacteria were then heat fixed by gently running the substrates over a low flame.
Modified acid-fast staining protocol on SPCE substrate
We modified the standard acid-fast staining procedure for the SPCE based detection of acid-fast Mtb. A fluorophore solution of rhodamine b (RhB) was prepared in a mixture of water, phenol and glycerol in 5:2:15 ratios. A 0.5% hydrochloric acid-ethanol (HCl-EtOH) mixture was prepared for the acid-treatment/decolorizing process. The substrates coated with both acid-fast and non-acid-fast bacteria, were initially heated to melt the mycolic acid layer of the acid-fast biological samples. The substrates were then flooded with the RhB solution that was allowed to stand for three minutes. Excess RhB was washed off using distilled water and the biological samples were subsequently subjected to acid-alcohol treatment by flooding the slide with HCl-EtOH mixture for one minute. Following acid treatment, the slides were again washed with distilled water and air-dried.
SPCE measurements
Following the modified acid-fast staining protocol; the GLAG-C60 substrates coated with the acid-fast and non-acid-fast bacteria, were affixed to a hemi-cylindrical prism. As in a typical SPCE setup in the Reverse Kretschmann (RK) configuration,8 the sample affixed prism was mounted on a calibrated rotary stage, where a 532 nm c.w. green laser was used as the excitation source. The SPCE signals (@580 nm) arising from the RhB coated bacterial samples were recorded using a fiber optic detector. A schematic depicting the RK configuration for performing SPCE studies on the GLAG-C60 substrates coated with bacteria is shown in (Figure 1A & Figure 1B).
Fluorescence microscopy
As stated earlier, in this work, we present SPCE as a non-microscopic tool for the specific detection of acid-fast Mtb over the non-acid-fast bacteria (E. coli and S. aureus) that are coated on GLAG-C60 substrates (Figure 1). However, we have also performed fluorescence microscopy on these samples, to validate that the SPCE signals indeed originate from the acid-fast structures. Fluorescence microscopy also enables us to assess the sensitivity of the SPCE platform that can be deduced by observing the number of cells being detected in the view scope area.
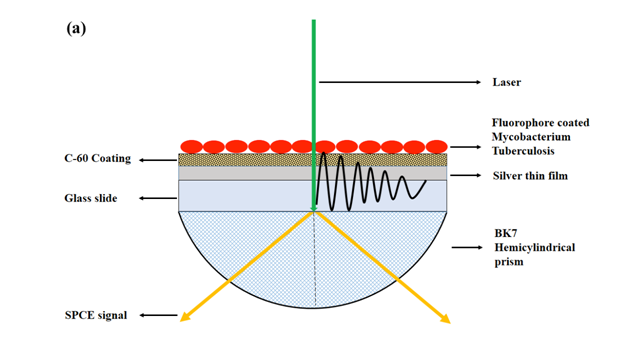
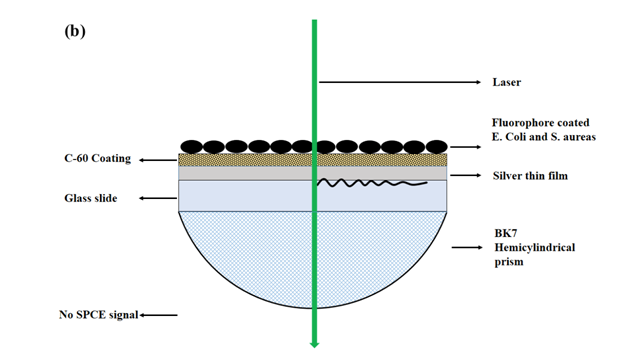
Figure 1 (a) A schematic depicting the use of GLAG-C60 SPCE substrates in the Reverse Kretschmann (RK) optical configuration, for the detection of acid-fast bacteria (Mtb). A laser beam directly excites the samples on the GLAG-C60 substrates and the SPCE signal is observed through the hemi-cylindrical prism. Bacteria samples are first coated, heat-fixed, and subsequently exposed to RhB. Acid-fact bacteria (Mtb) are resistant to acid-alcohol decolorizing and thus fluoresce strongly with a strong plasmon coupled signal; while non-acid-fast specimens. (b) E. coli and S. aureus do not exhibit any SPCE signal.
Figure 2A shows the images we obtained from performing fluorescence microscopy on the acid-fast M. tuberculosis; and the non-acid-fast E. coli and S. aureus samples. As evident in (Figure 2B), upon excitation with the green laser source, only the acid-fast Mtb cells were found to exhibit reddish-orange fluorescence upon staining and their characteristic rod-shaped morphology is clearly seen. On the other hand, the non-acid-fast E. coli and S. aureus cells did not fluoresce. This feature confirmed the high specificity of the acid-fast staining procedure towards only Mtb, which is due to the presence of long chain mycolic acid in the cell walls of Mtb.
On the contrary, the non-acid-fast E. coli and S. aureus bacteria present on the microscope slides (Figure 2C & Figure 2E); do not contain mycolic acid in their cell walls. They are hence easily decolorized upon acid-alcohol treatment, and do not fluoresce upon excitation (Figure 2D & Figure 2F).
While the microscopic examination (Figure 2) is highly specific and reveals the presence of Mtb, an early diagnosis of tuberculosis in RLS setting is still challenging due to the significantly lower number of Mtb cells observed in sputum samples, which may result in the generation of either weak or no signals in fluorescence microscopy. In this context, a chest X-ray scan and culturing of bacteria from sputum samples followed by fluorescence microscopy are prescribed; to definitively assess whether or not the patient is infected with TB. However, as described extensively before, the culturing usually takes a few weeks due to the extremely slow doubling rate of Mtb (16-20 hours per division), thereby subjecting the patient to an increased risk of disease progression. Moreover, fluorescence microscopes and X-ray scanners are often unavailable in RLS settings of low-and-middle income countries.
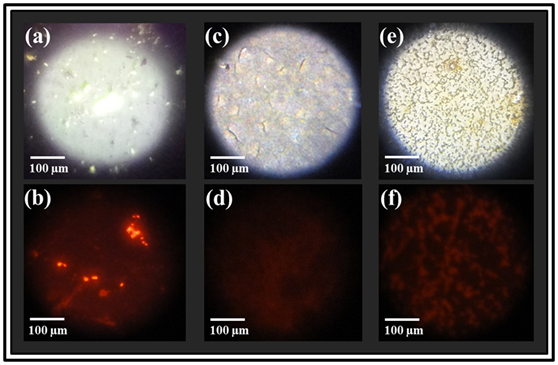
Figure 2 Photographs of the bacteria-coated AG-C60 substrates from fluorescence microscopy (top row) showing (a) Mtb cells, (c) rod shaped E. coli and (e) cocci clusters of S. aureus under white light illumination. The corresponding fluorescence photographs obtained using green laser excitation (l=532 nm) are shown in the bottom row where; only the acid-fast Mtb cells display bright orange fluorescence (@580 nm) revealing its characteristic rod shape morphology (b), while no fluorescence is observed from the non-acid-fast E. coli and S. aureus bacteria; (d) and (f) respectively.
SPCE based sensitive and specific detection of mycobacterium tuberculosis
The Ag-C60 SPCE sensing platforms enabled the highly sensitive and most importantly, the highly specific detection of M. tuberculosis, which is clearly reflected in (Figure 3). The fact that SPCE signals were produced only from the Mtb coated GLAG-C60 substrates irrefutably substantiates the high specificity of acid-fast staining to only the acid-fast Mtb cells. Furthermore, the SPCE signals that were generated, displayed exceptional signal-to-noise ratios, which further highlights high sensitivity that the Ag-C60 sensing platform provides. It is interesting to note that; the SPCE signals generated from Mtb coated Ag-C60 substrates @580 nm, corresponds to the reddish-orange color of fluorescence observed from the RhB stained, acid-fast Mtb cells in the fluorescence microscope image; (Figure 2). On the other hand, no fluorescence/SPCE signals were recorded from the non-acid-fast E. coli and S. aureus coated substrates, which strongly concurs with the results obtained from fluorescence microscopy imaging; (Figure 2C, Figure 2D & Figure 2F).
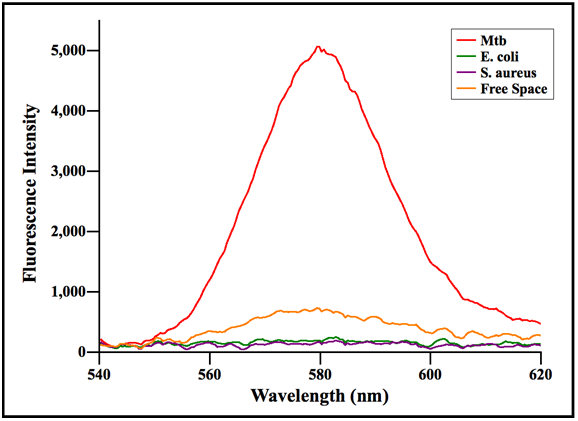
Figure 3 An intensity plot of fluorescence signals, generated from bacteria coated GLAG-C60 substrates, versus wavelength. It is clear that only the Mtb coated substrates generated strong SPCE signals highlighting the specificity of acid-fast staining to Mtb cells. Conversely, the non-acid-fast E. coli and S. aureus coated substrates displayed negligible fluorescence.
SPCE based fluorescence enhancement:
The characteristic features of SPCE signal profiles are;
Angularity of SPCE Signals: Indisputable evidence for fluorescence-plasmon coupling interactions was also observed in the form of strongly directional fluorescence signals that were generated on either side of the normal to the prism; in coherence with the theory of SPCE signal generation [4,8]. The angularity or directionality of SPCE signals, produced from the acid-fast Mtb coated GLAG-C60 substrate is represented in the form of a polar plot in (Figure 4).
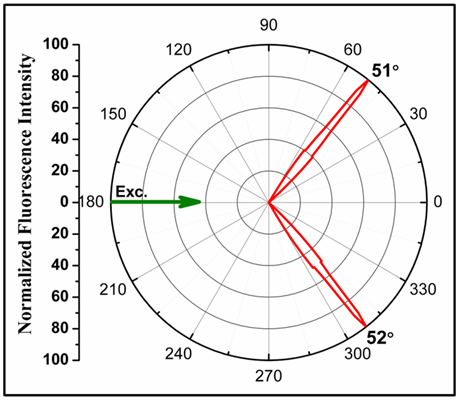
Figure 4 Radial plot depicting the highly directional angular distribution of SPCE signals, generated from Mtb coated GLAG-C60 substrates.
Ultra sensitivity of the Ag-C60 SPCE platforms
Based on the results obtained from our SPCE studies (Figure 3), we can see that the Ag-C60 SPCE substrates enabled the detection of Mtb samples with extraordinary sensitivity. From the microscopy data (Figure 2B); we evaluated the number density of Mtb cells immobilized from the stock culture solution, on the GLAG-C60 substrates. After acid-fast staining, the number density of Mtb cells on the substrates was determined to be around 90-100 Mtb/mm2. Considering the area of a typical laser spot (used as the excitation source in SPCE) to be around ~0.78 mm2 (an equivalent diameter of 1 mm); the detection limit of the GLAG-C60 platform was determined to be around ~20 Mtb/mm2, which highlights the ability of the GLAG-C60 substrates to detect such extreme low numbers/concentrations of Mtb cells. In this regard, while traditional microscopy based detection involves culturing of Mtb that can take up to 6 weeks; the attribute of ultra sensitivity of Ag-C60 platforms can enable the detection of TB directly from the biological samples within an hour.
The ultra sensitivity meted out by Ag-C60 nano-biosensors, empowers the application of these high performance thin-film substrates for achieving the long sought after; sensitive, specific, rapid and early diagnosis of tuberculosis. Furthermore; SPCE which is a simplistic table top platform, can be easily incorporated into mobile hospital facilities as point-of-care (POC) devices. This is of immense service and benefit for people from rural areas of the under-developed and developing countries, where facilities for chest X-ray imaging, bacterial culturing, and fluorescence microscopy are not generally available.
In this study, we have successfully demonstrated the first-time fabrication and use of inexpensive SPCE platforms as novel point-of-care sensors, for the rapid and sensitive detection of the dangerously pathogenic Mycobacterium tuberculosis, which is of immense relevance to resource limited settings around the world. Furthermore, SPCE based sensing has eliminated the requirement for any sophisticated and cumbersome optical configurations, or elaborate microscopic evaluation, highlighting the simplicity of this novel technique. The ability to detect Mtb at an early stage (~20 Mtb/mm2), using the low cost Ag-C60 substrates is a critical advance to the current fluorescence-based methods, which are limited due to the inherent low-sensitivity associated with isotropic fluorescence.
None.
The author declares no conflict of interest.

©2016 Kamisetti, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.