International Journal of
eISSN: 2573-2838


Review Article Volume 7 Issue 3
Department of Studies in Chemistry, Vijayanagara Sri Krishnadevaraya University, India
Correspondence: Lokesh Koodlur Sannegowda, Department of Studies in Chemistry, Vijayanagara Sri Krishnadevaraya University, Vinayakanagara, Cantonment, Ballari-583105, Karnataka, India
Received: May 12, 2021 | Published: May 26, 2021
Citation: Nemakal M, Sannegowda LK. Hybrid composites based on phthalocyanine and carbonaceous materials for sensing applications: a review. Int J Biosen Bioelectron. 2021;7(3):84-89. DOI: 10.15406/ijbsbe.2021.07.00218
The hybrid composite materials have a profound interest due to the synergetic combination of the properties to yield significant enhancement in the desired properties. Hybrid composites of phthalocyanine with carbonaceous materials are known to have surprisingly superior catalytic and sensing response. This review provides a brief discussion on the design and construction of phthalocyanine based hybrid composite for sensing environmentally important pollutants and biomolecules. These composite material-based electrochemical sensors are applied for the nanomolar to millimolar level detection of various environmental pollutants, toxic molecules, heavy metals and bio-active molecules. The hybrid systems are sensitive, selective and yield reliable responses. Furthermore, possible catalytic sensing mechanism at the hybrid sensor system has been discussed.
Keywords: metal phthalocyanine, hybrid composite, carbonaceous materials, electrochemical sensors
A sensor is an analytical tool that provides a highly sensitive and selective response for an analytic species or bio-origin substance. These sensors find wide scope and enormous applications in the field of environmental science, pharmaceuticals, food processing and preservation industries, process industries, securityand protection, robotic automation, etc.1–3 Among the various types of sensors, electrochemical sensors have unique characteristics.4,5 These sensors are specific for screening and monitoring of analytes with fast, reliable, easy, portable, and cost-effective advantages. In addition, electrochemical sensors are robust and specific in the monitoring of various analytes.5 Electroanalytical techniques such as potentiometric, chronoamperometric (CA), and cyclic voltammetric (CV), differential pulse voltammetry (DPV), linear sweep voltammetry (LSV) based sensor systems are employed for the detection of analytes at low concentration. The mechanism of sensing includes the interaction between analyte and working electrode pinhole surface to induce redox reaction. Inorder to decrease the overpotential, fouling effect and improve the response, virgin electrodes are modified with appropriate redox material or stimulant. Different redox-active materials like nanoparticles, metal oxides, carbonaceous materials, polymers, macrocycles and organic compounds have been employed for functionalizing the electrode surface.5,6 Out of these, N4 macrocycles like phthalocyanines (Pc) are interesting because of their unique properties and in addition, their properties can be altered or tuned by substituting different functional groups or metal ions.7,8 Furthermore, the properties and catalytic activity of this organic-based macrocycles can be enhanced by preparing an organic hybrid composite of Pc with carbonaceous materials. These hybrid composites are easy to design and exhibit improved activity for different applications.6
Phthalocyanine as sensor material
Varieties of physicochemical detectors or transducing microsystems are used to increase the sensitivity and response. Bare electrodes are known to have higher overpotential and lesser response for the analytes. Hence, bio-inspired molecules such as porphyrins and Pc’s are fascinating and have structural similarities with chlorophyll and hemoglobin9,10 Metal phthalocyanine (MPc) (Figure 1) macrocycle has a central cavity and accommodates different metal ions through coordination bond. The geometry of metal-free phthalocyanine is square planar, i.e. D2h symmetry. If the planarity is maintained on the attachment of metal ion inside the phthalocyanine cavity, the symmetry increases from D2h to D4h. Moreover, Pcs can be tailored with peripheral, axial or double-decker substitution to acquire improved stability, redox-behaviour, planarity, conjugation, etc. For electrochemical applications, MPc has to be immobilized on the electrode surface as a thin film either by Langmuir-Blodget,11 electropolymerization,12 chemical vapor deposition (CVD), drop-coating/casting or layer-by-layer deposition,13 electro-sputtering, self-assembled monolayers (SAM’s),14 or sublimation technique, etc. The catalytic activity of MPc can be enhanced by preparing organic hybrid composite with conducting and high surface area carbonaceous molecules, Figure 2. This two-component hybrid composite material possesses superior properties. The carbonaceous materials have larger surface area, porosity, and more active site.15 Various carbonaceous materials like carbon nanoparticle (CNP), carbon nanotubes(CNT), multiwalled carbon nanotubes (MWCNTs), reduced graphene oxide (r-GO), graphene (Gr), etc., have been employed in the fabrication of composites.
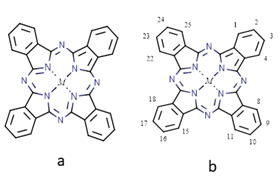
Figure 1 A) Structure of metal phthalocyanine (Pc) (M=Co, Cu, Ni, Zn, Fe, etc.). (B) IUPAC nomenclature numbering scheme for the phthalocyanine core.
Fabrication of sensors
The hybrid composite of MPc complex with carbonaceous material has been prepared by simply sonicating in a suitable solvent like DMF, DMSO, CH2Cl2 or isopropyl alcohol or with grinding.
Hybrid composite materials for electrochemical sensing
The sensing efficiency of the composite can be enhanced by employing varying ratios of the components. The schematics involved in designing and application of composite material for sensing are illustrated in Figure 3.
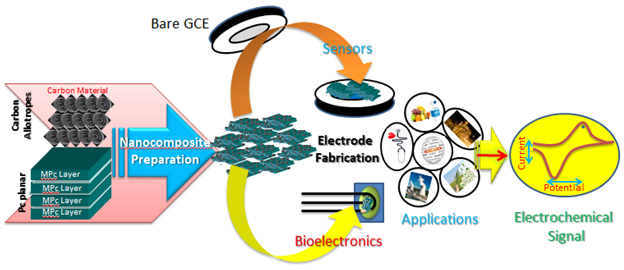
Figure 3 Schematics involved in the fabrication of composite material and their applications in electrochemical sensing.
The enhancement in sensing ability of hybrid composite can be explained as follows:
Hybrid of PdPc with MWCNT has been evaluated for the simultaneous detection of biomolecules like ascorbic acid (AA), uric acid (UA) and dopamine (DA)19–21 at nanomolar concentration using cyclic voltammetry and amperometry. CoPc in presence of MWCNT provides desirable conditions for electron transport and enhances the anodic peak current and facilitates oxidation of AA.21 The significant increase in the catalytic activity of the composite is mainly because of synergistic and cooperative action between CoPc and MWCNT.21
CoIIPc–MWCNT/GC → CoIPc–MWCNT/GC + 1e-1 --------------- (1)
2CoIPc–MWCNT/GC + AA(anlyte) → 2CoIIPc–MWCNT/GC + AA(Oxidised product) +2H+----(2)
Non-precious and nonenzymatic MPc’s of Fe+2, Co+2, Cu+2, Ni+2 and other peripheral substituted complexes along with carbonaceous materials have been successfully used for the detection of glucose in food and biological specimens.22 Even, these hybrid systems have been employed for the sensing of hydrogen peroxide.23 The mechanism of glucose detection involves the oxidation of glucose to form gluconolactone at phthalocyanine ionic liquid graphene composite electrode22 and the steps of oxidation process are as follows:
[CoIIPc/IL/G/SPCE/PADs]H2O+Glucose(Analyte)→[CoIIPc/IL/G/SPCE/PADs]Glucose(Analyte)+H2O -----------------------(3)
[CoIIPc/IL/G/SPCE/PADs]→[CoIIIPc/IL/G/SPCE/PADs] + 1e-1-------------- -(4)
[CoIIPc/IL/G/SPCE/PADs]Glucose(Analyte)+[CoIIIPc/IL/G/SPCE/PADs]→ 2[CoIIPc/IL/G/SPCE/PADs] + Gluconolactone(oxidized product)+ 1e-1+2H+------------------- (5)
Aminoacids like L-arginine, cysteine etc. are the building blocks of proteins and these aminoacids have been detected at micromolar concentration level instantly with high sensitivity.24,25 L-glutathione (L-GSH) and its disulfide (GSSG) have been detected in physiological pH of 7.4 by the utilization of cobalt phthalocyanine (CoPc) on ordinary pyrolytic graphite electrode. The remarkable result is that the electrocatalytic activity of adsorbed CoPc for the oxidation of GSH at physiological pH is effective. It occurs following a mechanism as reported in Eq. (6-10).26
R-SH → R-S- + H+ --------------------------------------(6)
R-S- + CoIIPc- → [CoIIPc-S-R]- -------------------------(7)
[CoIIPc-S-R]- → [CoIIPc-S-R] +1e----------------------- (8)
[CoIIPc-S-R]→CoIIPc + R-S*---------------------------- -(9)
R-S* + R-S*→ R-S-S-R---------------------------------- (10)
Heavy metal ions like Hg+2, Pb+2 and Cr+6 are highly hazardous and cause adverse effects on human beings and the ecosystem even at low concentrations. These heavy metals have been detected at nanomolar concentration using Pc-CNP derived composite material by amperometry.27–30 Not only monitoring, even the detoxification of such toxic metals can be done either by reduction or oxidation of the analyte at the composite electrode. The composite materials have also been employed for the detection of other heavy metals namely Cu, Cd, As, Cs, etc.29–30 Organic hybrids have been widely used in the detection of triod and tetaroids of purines and pyrimidines.31 These biomolecules help in the formation of DNA by using adenine, guanine, thymine, cytosine and uracil. These nitrogenous bases have been analyzed in biological DNA specimens.32–34 Caffeine, nicotine and tannic acid are biomolecules and their content in leaf extract and tea powder has been detected by the application of a hybrid catalyst system.35 Furthermore, pharmaceutical drug molecules like paracetamol has been analyzed along with their known co-existing impurities using hybrid sensor.36,37 Even salicylic acid, vanillin, 4-aminophenol,36 bis-phenol,38 4-nitrophenol,39 para-phenylenediamine(PPDA) and, ortho-phenylenediamine (OPDA), etc., were also detected using Pc based composites. The water and environmental pollutants such as nitrite/nitrate,40–42 hydrazine,43 phenol (hydroquinone, catechol, and resorcinol),44 pesticides like dichloro phenol.45,46 thiols, and substituted thiols,47,48 and others have been detected selectively at nanomolar or micromolar concentrations using Pc based hybrid composites.49 Composite of cobalt (II) tetramethyl-quinoline oxy bridged phthalocyanine with carbon nanoparticles was applied for the detection of nitrite at nanomolar level. First nitrite forms adduct with Pc complex and then the oxidation process takes place with the support of electroactive and conductive CNP.40–42
2(CoIIPc) →2(CoIIIPc) -----------------------------------(11)
2(CoIIIPc) +2(-O-N=O) →2(CoIIIPc-O-N=O) -----------(12)
2(CoIIIPc-O-N=O) → 2(CoIIPc) + 2NO2 + 2e---------- -(13)
2NO2 + H2O → NO2-+ NO3-+ 2H+---------------------- (14)
The electrooxidation of thiols and substituted thiols at iron phthalocyanine electrode is as presented in equation 15-18. Firstly formation of adduct takes place and subsequently free radicals of thiolate ions are produced and finally disufide derivatives are formed at iron phthalocyanine modified graphite electrodes.48
R-SH + OH-→ R-S- + H2O----------------------------- (15)
R-S- + FeIIPc→ [FeIPc-S-R]- ----------------------- ----(16)
[FeIPc-S-R]-↔ FeIIPc + R-S*+1e----------------------- (17)
R-S* + R-S*→ R-S-S-R------------------------------- -(18)
The amperometric step current response for hydrazine detection at octabenzimidazole Pc embedded rGO composite is superior compared to the bare electrode and the response stabilizes within 3 seconds (Figure 4) which is an important parameter in the sensor technology. The catalytic mechanism for the hydrazine oxidation can be illustrated as shown in Figure 5 and the path of the oxidation process is as in (eq. 19-22):43
N2H4 → N2+ 4H+ + 4e— ------------------------------- -(19)
poly-Co+2Pc-rGO+ N2H4 + OH— → [poly-Co+1Pc-rGO]— (N2H3)+ + H2O + e— -------(20)
[poly-Co+1Pc-rGO]— (N2H3)+ + OH— →poly-Co+2Pc-rGO+(N2H2). + H2O + e— -------(21)
(N2H2)— + 2OH— → N2 + 2H2O +2e— ----------------- (22)
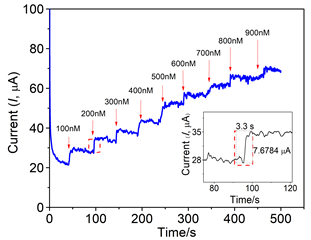
Figure 4 Chronoamperometric response for hydrazine sensing at MPc-rGO composite electrode. Inset: magnified current response for each addition of hydrazine.

Figure 5 Mechanism for the oxidation of hydrazine (HZ) at the cobalt (II) Pc polymeric film-rGO composite electrode.43
Table 1 explains the analytical data for the detection of different analytes at various composite electrodes.The synergetic combination of MPc organic frame with carbon allotrope enhances the response of analytical and bioanalytical species and the oxidized or reduced form of MPc are responsible for the catalytic activity. The composite provides reliable, reproducible and fast response which makes them attractive materials for sensing applications.
|
Electrode |
Electrolyte |
§Analytes |
Sensitivity |
Range (μM) |
LOD(μM) |
Ref. |
|
aGCE/MWCNTs/PdTAPc |
0.25 mol L−1 H2SO4 |
AA |
1.0149μA μM−1 cm−2 |
3–24 |
1.0 |
[19] |
|
DA |
1.9303 μA μM−1 cm−2 |
2–16 |
0.6 |
|||
|
UA |
0.4328 μA μM−1 cm−2 |
5–40 |
1.5 |
|||
|
bN-G/NiTsPc/GCE |
0.1M phosphate buffer (pH=7) |
DA |
0.926μA μM−1 cm−2 |
0.1—200 |
0.1 |
[20] |
|
cCoPc–MWCNTs/GC |
0.1M phosphate buffer (pH=7) |
AA |
- |
10 -2600 |
1.0 |
[21] |
|
dCoPc/IL/G/SPCE/PADs |
0.1 M NaOH containing |
Gl |
- |
0.01−1.5 &1.0−5.0 |
0.67 |
[22] |
|
eGCE/GO/poly(CoTBIPc) |
phosphate buffer (pH=7) |
H2O2 |
1.0004 μA μM−1 |
2–200 |
0.6 |
[23] |
|
fRGO-pTACoPc |
phosphate buffer (pH=7.4) |
L-cys |
10.19μAμM−1 cm−2 |
0.05–2.0 |
0.018 |
[25] |
|
gCoPc-OPG |
0.1 M NaOH containing(pH= 7.4) |
GSSG |
23 μA μM−1 |
0.08-1 |
- |
[26] |
|
GSH |
11.5 μA μM−1 |
- |
||||
|
hGCE/poly(CoTABImPc) |
0.1M phosphate buffer (pH=7) |
Hg(II) |
3.7389 μA μM−1 cm−2 |
10–400 |
3 |
[27] |
|
iPcIrCl/GCE |
0.1 MHCl |
Cr(VI) |
- |
0.05 – 2.5 |
0.018 |
[28] |
|
jCoTEIndCAPc/MWCNTs/GC |
phosphate buffer (pH=7.0) |
Cd(II) |
- |
100-1000 nM |
5 nM |
[30] |
|
Pb(II) |
- |
100-1000 nM |
3 nM |
|||
|
kWCNTs–PNF film modified GCE |
phosphate buffer (pH=7.4) |
GU |
12.22 mA M−1 cm−2 |
0.1-8.5 mM |
98.56 |
[32] |
|
AD |
89.31mA M−1 cm−2 |
0.01-3.9 mM |
9.2 |
|||
|
TH |
41.92mA M−1 cm−2 |
0.02-7.7 mM |
19.3 |
|||
|
laPcCo/CNT hybrid/GC |
phosphate buffer (pH=7) |
4-AP |
- |
0.5-1000 |
0.3 |
[35] |
|
mGCE/CNP-CoTBTCAPc |
phosphate buffer (pH=7) |
4-AP |
888.7μA μM−1 cm−2 |
0.1 - 0.9 |
0.030 |
[36] |
|
nGCE/CoTATPAPc |
phosphate buffer (pH =7) |
PA |
3.0208 mA nM−1 cm−2 |
20-180 nM |
4.1 nM |
[37] |
|
4AP |
2.9728 mA nM−1 cm−2 |
20-180 nM |
4.2 nM |
|||
|
oGNS-FePc/GCE |
phosphate buffer (pH=7) |
4-NP |
1.68μA μM−1 cm−2 |
100-700 |
10 |
[39] |
|
pPoly(TazoCoPc)/CNP/GCE |
phosphate buffer (pH=7) |
Nitrite |
137.2μA μM−1 |
0.02–1 |
0.006 |
[40] |
|
qCoTM-QOPc/CNP/GCE |
phosphate buffer (pH = 7) |
Nitrite |
1.237μA nM−1 cm−2 |
0.1-350 |
0.033 |
[42] |
|
rGCE/r-GO/poly(CoOBImPc) |
0.1 M NaOH |
HZ |
0.3696μA nM−1 cm−2 |
0.1–0.9 |
0.033 |
[43] |
|
sGCE/poly-CoPc |
pH 3.8 acetate buffer |
2,4-DCP |
- |
0.5–10 |
0.15 |
[46] |
Table 1 Analytical parameters for the detection of various analytes atcomposite electrodes of MPcembedded with carbonaceous materials
aGCE/MWCNTs/PdTAPc: self-assembled palladium tetra amino phthalocyanine modified with multiwalled carbon nanotubes on glassy carbon electrode;bN-G/NiTsPc/GCE: nickel tetrasulfonated phthalocyanine functionalized nitrogen-doped graphene nanocomposites;c CoPc–MWCNTs/GC: Cobalt(II) phthalocyanine–multi-walled carbon nanotubes modified glassy carbon; dCoPc/IL/G/SPCE/PADs: paper-based analytical device (PAD) modified on a screen-printed carbon electrode with cobalt phthalocyanine, graphene and an ionic liquid;eGCE/GO/poly(CoTBIPc): graphene oxide modified cobalt phthalocyanine substituted with tetrabenzimidazole (CoTBIPc) moieties on glassy carbon electrode;fRGO-pTACoPc: reduced graphene oxide polymeric tetra amine cobalt phthalocyanine; gCoPc-OPG: Cobalt phthalocyanine on pyrolytic graphite disk ;hGCE/poly(CoTABImPc): glassy carbon electrodemodified by electropolymerisedphthalocyanine film;iPcIrCl/GCE: iridium tetra [4-o-tolylsulfanyl] chloride phthalocyanine (PcIrCl)modifiedglassy carbon electrode; jCoTEIndCAPc/MWCNTs/GC: tetra–ethyl-1H-indazol-3-carboxamide Cobalt (II) Phthalocyanine (CoTEInd-CAPc) with MWCNTs, decorated on glassy carbon electrode (GCE);kMWCNTs–PNF film modified GCE:multi-walled carbon nanotubes incorporated with poly(new fuchsin) composite film modified glassy carbon electrode;laPcCo/CNT hybrid/GC: tetra-b-[3-(dimethylamine) phenoxy] phthalocyanine cobalt(II)/multiwalled carbon nanotube hybrid modified glassy carbon electrode;mGCE/CNP-CoTBTCAPc: glassy carbon electrode modified with carbon nanoparticlesphthalocyaninesensors;nGCE/CoTATPAPc: glassy carbon electrode modifiedcysteine substituted cobalt phthalocyanine;oGNS-FePc/GCE: graphene nanosheets (GNS) decorated iron phthalocyanine (FePc) film modified electrode; pPoly(TazoCoPc)/CNP/GCE: tetraazo-bridgedcobalt phthalocyaninepolymercarbon nanoparticles modified glassy carbon electrode; qCoTM-QOPc/CNP/GCE: Cobalt (II) tetra methyl-quinoline oxy bridged phthalocyanine carbon nanoparticles modified glassy carbon electrode.rGCE/r-GO/poly(CoOBImPc): reduced graphene oxide modified polymeric cobalt octabenzimidazolephthalocyanine;and sGCE/poly-CoPc: glassy carbon electrode modified polymeric cobalt sheet phthalocyanine.
Analytes:AA-Ascorbic acid; DA-Dopamine; UA- Uric acid; Gl-Glucose, H2O2- Hydrogen peroxide; L-Cys-L-cysteine;L-GSSG- disulfide of glutathione; L-GSH- L-glutathione; 4AP-4-aminophenol; PA- Paracetamol; 4-NP-4-nitrophenol; Hg(II)-mercury; Cr(VI)-chromium; Cd(II)- Cadmium; P b(II)-Lead; GU-Guanine; AD-Adenine; TH-Thymine; HZ-Hydrazine; and 2,4-DCP-2,4-dichloro phenol.
This mini-review gives an insight into the fabrication and designing of metal phthalocyanine-carbonaceous material-based hybrid sensors. MPc’s are well-known for their superior thermal stability, chemical inertness, higher conjugation and redox-active behaviour. The catalytic efficiency of the MPc thin layer on the working electrode can be improved by forming organic hybrid composites with either GO, rGO, functionalized CNP, MWCNT’s, Ketjen Black, CNP, etc. The carbon-derived materials enhance the catalytic and electronic properties. The two-component hybrid composite acts as a potential and promising material for the detection and determination of various pollutants and bio-active materials. These hybrid materials can detect the species at trace and nanomolar concentrations with higher sensitivity and selectivity for a specific analyte. Enhanced activity is derived from the increased surface area and ultrahigh porosity of the electrodes for surface reactions.
Authors acknowledge research grant from DST-SERB, Govt. of India No. SERB/F/9388/2016-17. We thank CSIR grant No. 01(2915)/17/EMR-II. We also thank DST-FIST (SR/FST/CSI-003/2016) and VGST –KFIST (KSTePS/VGST-KFIST(L1)/2017/267) grants.
There are no conflicts of interest to declare.

©2021 Nemakal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.