International Journal of
eISSN: 2573-2838


Research Article Volume 4 Issue 6
Saitama University, Japan
Correspondence: Yuki Hasegawa, Graduate school of Science and Engineering, Saitama University, 255 Shimo-Okubo, Sakuraku, Saitama, Japan, Tel +81 48 858 3889
Received: November 26, 2017 | Published: December 5, 2018
Citation: Murohashi F, Uchida H, Hasegawa Y. Evaluation of photosynthetic activity by bioelectric potential for optimizing wavelength ratio of plant cultivation light. Int J Biosen Bioelectron. 2018;4(6):28110.15406/ijbsbe.2018.04.00141
It is necessary to provide the growth rate of crops for improve efficiency of plant factory. It is well known that the growth rate of plants has a strong relationship with the wavelength of light and there is an appropriate wavelength ratio depending on the variety of plants. In this study, we attempted to evaluate physiological activity such as photosynthesis using plant bioelectric potential for optimizing the wavelength ratio of plant cultivation light source. We employed three varieties of strawberry (Elan, Nyoho and Akihime) as a sample plant, and these bioelectric potential responses were measured and compared when changing the wavelength ratio of cultivation light source which included blue (475 nm), green (525 nm), and red (660 nm) LEDs. The results showed the different responses to various wavelength ratios of light for each variety. It was also observed that bioelectric potential response has a correlation with CO2 consumption accompanying the photosynthetic activity change due to the wavelength ratio of the irradiation light.
Keywords: plant bioelectric potential, photosynthetic activity, wavelength ratio of irradiation light, plant factory, strawberryToday, facilities that product crops under an artificial environment such as a plant factory have increased attention concerning the food crisis caused by global population growth and reduction of agricultural land. Plant factory can control the cultivation environment such as temperature, humidity, nutrient solution, CO2 concentration, light and so on, in a closed space. Therefore, it is expected to stably product crops without influence of weather or season to be combined with a multistage cultivation shelf and hydroponic cultivation.1, 2 However, it has some serious issues such as reducing installation and operation costs for environment control3 and improving efficiency of production crops.
As is well known, one of the important elements for plant growth is light irradiation which is essential element for photosynthesis. Oxygen and sugar are produced from carbon dioxide and water using light energy, and high energy substances that can be formed during photosynthesis are utilized for growth. The light absorption spectrum of chlorophyll which is one of the photosynthetic pigments, has two peaks around blue (450nm) and red (660nm) regions.4 Recently, LEDs are used instead of a fluorescent lamp conventionally used as light source for plant cultivation. LEDs have some advantages to improve the cultivation efficiency such as power saving, low heat generation, long life and a narrow spectral band matching an absorption spectrum of the photosynthetic pigment.3,5–8 Many papers reported that the wavelength of the irradiated LEDs related with the growth rate of the crops, and the light source wavelength and that combination or ratio are important for plant growth.8 Especially, the effects of blue and red lights have been studied in various species such as strawberry9–12, rose13, lettuce14–19 and so on. Thus, it is important to appropriately set the wavelength ratio of the cultivation light sources according to the crops and varieties to be cultivated.
Therefore, we focused on plant bioelectric potential as one of speaking plant approach (SPA) techniques20 and the promising method to evaluate the physiological activities of plants inexpensively, nondestructively and in real time. Plant bioelectrical potentials are generated by the ion concentration differences between inside and outside of the plant cell membrane and are closely related to the physiological activities of plants.21,22 Plant bioelectric potentials are known to exhibit a characteristic response to irradiation or interruption of light. In addition, our previous studies clarified that there is a positive and strong correlation between the potential response and CO2 consumption when
changing the light intensity of LEDs or temperature.23–25 However, it has not fully clarified that the relationship between the plant bioelectric potential responses and light source wavelength and that combinations. In this study, the bioelectric potential responses of three varieties of strawberry were measured when various wavelength ratios of light included blue (475nm), green (525nm) and red (660nm) LEDs irradiate. And then, we evaluated the relationship between the responses and the photosynthetic activities and examined that plant bioelectric potential measurement can be a useful method for determining light quality of light source for cultivation.
Sample plant
Three varieties of strawberry (Fragaria×ananassa, cultivar: Elan, Nyoho and Akihime) were employed as a sample plant. The strawberry belongs to the family Rosaceae, genus Fragaria, and is among the most widely cultivated and consumed fruit all over the world.26 So, it has expected to be produced in artificial facilities all the year round. However, strawberry cultivation in the artificial facilities such as plant factory requires much energy and cost compared with open field cultivation.27 Therefore, we consider that strawberry is one of the suitable crop for experiments aiming at high efficiency of cultivation in plant factory.
Measurement environment and conditions
In our experiment, we used a plant growth chamber (ESPEC, BAC-130H) which can control temperature, humidity and light conditions. The nutrient solution (OAT Agrio, OAT house, pH: 5.5 to 6.0, EC: 1.1 to 1.2mS) was placed in clear container to resemble the environment of hydroponic culture and the roots were immersed and allowed to stand in the closed vessel where under the LEDs panel. The light source that used in experiment is a plant growing LEDs panel. The light intensity was evaluated by the photosynthetic photon flux density (PPFD) which is one of the light intensity unit using agricultural field, it is measured light intensity of wavelength with 400 to 700nm that can be absorbed by chlorophyll of plant using a photon counting meter (LI-COR, LI-250). PPFD was measured at the center of the leaf surface and set to be constant for each measurement condition. First, we examined differences in bioelectric potential response to monochromatic light for each type of strawberry (Condition I). In Condition I, the PPFD at the time of measurement at Elan or Nyoho and Akihime are different (Table 1), due to the difference in height of the LED panel and the leave of sample. Next, the Experimental conditions (Condition II - VII) to compare the wavelength ratio with blue, green and red lights is shown in Figure 1. Conditions II and III show the difference in bioelectric potential response due to the red-blue ratio of the light source, Conditions IV to VI show changes in bioelectric potential response when adding green light to red or red-blue lights, Condition VII shows the difference in bioelectric potential response due to the green-red ratio. For Conditions IV to VI, the ratio of green light to the whole irradiation light is set to 0, 25%, 50%, and the wavelength ratio of red [%]: blue [%] is set to 100: 0, 75: 25, 50: 50 for the remaining components. Conditions II to VII were conducted using only Elan.
Varieties of strawberry |
PPFD of irradiation light [µmol/m2s] |
Elan |
35 |
Nyoho |
43 |
Akihime |
43 |
Table 1 PPFD of irradiation light in Condition I
Plant bioelectric potential measurement
Figure 2A&2B show the schematic diagram of our measurement system of plant bioelectric potential and the image of LEDs panel (CCS, ISL-305X302-RFGB) used in our experiment. The LEDs panel included blue (475nm), green (525nm), red (660nm) and far red (735nm) and arranged at 1: 1: 3: 1. The needle type electrode of electroencephalogram made of stainless steel (Nihon Kohden, NE - 224S) was used in measurement. An electrode inserted to the stem of sample plant as a reference electrode and another electrode inserted to the leaf vein on the back of a leaf. The DC voltage between these electrodes measured with high input impedance digital multi meter (> 1 GΩ, ADVANTEST, R6552), and it was recorded on the PC at a sampling interval of 5 s. The measurement was conducted after 24 hours or more since the electrode was inserted to avoid the influence of the electrode insertion as much as possible. The sample plant was keeping in the dark state for 60 minutes, and then the plant potential was measured with the light irradiation and interruption repeatedly per 30 minutes for 5 hours under each light condition described in ‘Measurement environment and conditions’. Figure 3 shows a typical bioelectric potential response, definition of response value Von and some distinctive peaks. Peaks n1 to n3 represent the extreme values of the bioelectric potential response at light irradiation. Peaks n1 and n2 were confirmed in all measurements, but n3 was not observed in some measurements. Therefore, in this study, Von was defined from peaks n1 and n2 as a parameter of the physiological activity evaluation, and the mean value from five Von and that error bar giving the standard deviation were used to evaluate.
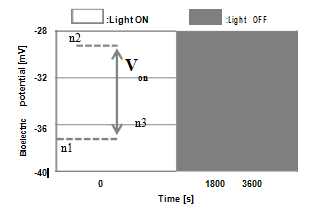
Figure 3 Typical potential response to light irradiation or interruption and definition of evaluation parameter Von.
CO2 concentration measurement
The CO2 concentration change in the 22.4 L closed vessel containing the sample plant was measured using a non dispersive infrared (NDIR)-type CO2 analyzer (LI-COR, LI-840) to investigate the relationship between the wavelength ratio of the light irradiation and the photosynthetic activity. The measurement result is as shown in Figure 4. The CO2 concentration increases while the light interruption and decreases when the light irradiated. These results are due to CO2 production by respiration and CO2 consumption by photosynthesis. In addition, under the light irradiation, it is mixed two kinds of phenomenon that are absorbing CO2 by the photosynthesis and releasing CO2 by the respiration at the same time. Thus, it is necessary to add to measured CO2 consumption and the increment in CO2 concentration by the respiration to obtain CO2 consumption of true photosynthesis. We considered that the respiration activity under the light irradiation as the same activity with the light interruption period just before the light irradiation, so a dot line was drawn in Figure 4 as the increase by the respiration. The intersection between this dot line and the vertical axis at the next light interruption is the concentration of CO2 in the container that arrived when photosynthesis was not performed. The difference between the CO2 concentration at this time and the CO2 concentration reached at the light irradiation obtained by actual measurement was taken as true CO2 consumption by true photosynthesis. From this CO2 consumption value, the CO2 consumption rate was calculated from an equation (1) using the molar mass of CO2 (44×103 mg/mol).
Monochromatic light irradiation
Figure 5 shows the bioelectric potential responses when monochromatic lights of blue (475nm), green (525nm), and red (660nm) are irradiated to each sample of Elan, Nyoho and Akihime. The bioelectric potential responses to the light irradiation and interruption were observed under any color of light. Figure 6 shows Von when irradiating light of each color to the sample plant. Von for each monochromatic light differs between each strawberry, but the difference in response to each color of light was confirmed. Elan and Nyoho had large bioelectric potential responses to red light and the lowest responses to blue light. Elan had a big difference in response to green light and blue light, but the difference between green and blue of Nyoho was small. Compared to Elan and Nyoho, Akihime had almost same response no matter what wavelength it was irradiated. From these results, it is considered that the difference of potential response has related to preferred light wavelength of each variety of strawberry.

Figure 5 Bioelectric potential waveform of each variety to monochromatic light irradiation. The gray background areas represent in light interruption period.
Changes in Red-Blue ratio of light irradiation
In the experiment of monochromatic light irradiation, Elan shows conspicuous difference when irradiated each wavelength. Therefore, we used Elan to investigate the optimal red-blue ratio of the light irradiation and measured the bioelectric potential responses in Condition II. In Figure 7A, the response to 100 % blue light was smaller than the including red light, and Von also increased as the proportion of red light increase. Therefore, the measurement in Condition III was carried out with the proportion of red light set to 50% or more and the proportion of blue light changed by 10%. Figure 7B shows a comparison of Von in Condition III. This figure shows similarly to the measurement in Condition II, Von is the largest when the red light proportion is 100%, but Von when the red light is 60% is the second largest, and the difference of Von as a whole is small. From these results, Von varies depending on the red-blue ratio of the light source and tends to increase as proportion of red light increase. However, the difference in Von when the red light is 50% or more tends to be small. The Red-Blue ratio of light with absorption spectrum of chlorophyll is important for the growth of plants. To consider with the result in previous section, it was thought that Elan has a great influence with red light on growth. Figure 8 shows the relationship between red-blue ratio of light irradiation and CO2 consumption rate in condition II using another sample of Elan. As with the potential response, the larger proportion of red light was the higher consumption rate of CO2. These results indicate when the proportion of red light is larger, photosynthetic activity is higher and the change of Von has related to the change in photosynthetic activity.
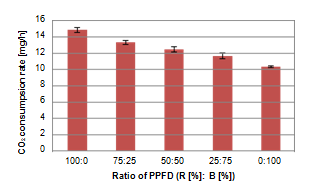
Figure 8 Difference in CO2 consumption rate when changing Red-Blue ratio of irradiation light (ConditionⅡ).
Changes in Red-Green-Blue ratio of light irradiation
Finally, the bioelectric potential responses were measured and compared when green light was added while keeping whole light intensity and red-blue ratio of light constant. Figure 9 shows a comparison of Von in Conditions IV, V and VI. Von was increased by adding green light respectively and was the largest when the ratio of green light was 25% under all conditions. From this measurement and the result in previous section, it was reconfirmed that Von at the light irradiation with only red and blue without adding green light was the largest at 100% red. Regarding Figure 9C, the potential response became the almost same regardless of the wavelength ratio of the light irradiation. These results indicate that the photosynthetic activity is reduced due to the reduction of red light in the whole irradiation light, and the activation of photosynthesis by the adding green light is canceling out. However, we considered that the optimum red-green ratio exists because Von increases when adding green light to red light. Therefore, changing the proportion of green light more finely under the condition that red light is 50% or more and Von was compared. Figure 10 shows a comparison of Von in Condition VII. When increasing the ratio of green light to the whole light irradiation, Von reached the largest value at 30% green. From Figure 9&10, Von was higher than only monochromatic red light when the optimized red-green ratio of light irradiation. Thus, it was clarified that Elan is most actively photosynthesized when red-green ratio of light irradiation is 70 %: 30 %. Furthermore, by using another sample, CO2 consumption was measured and compared when changing the proportion of red and green in the PPFD of the irradiation light by 25%.The results are shown in Figure 11. In this Figure, it is seen that CO2 consumption increases when adding green light to monochromatic red light. Especially, the changes of CO2 consumption in Figure 11 Von in Figure 9A and 10 from 0 to 50% green light had similar trend.
In the experiment of monochromatic light irradiation, bioelectric potential responses for each color differed for each strawberry variety, and the response of Akihime has no major differences to any color, whereas the responses of Elan and Nyoho increased in the order of red, green and blue. It was confirmed that differences of small physiological activities among varieties also appear in the response of bioelectric potential even for the same kind of plant. It is well known that the wavelength ratio of the cultivation light source is important for growth, however to compare the growth by irradiating light of various wavelengths to each plant or variety, enormous time and space are required. On the other hand, it is possible to acquire evaluation parameters for one kind of irradiation light in about 6 hours using our suggested technique; it is possible to evaluation the wavelength ratio suitable for the object in a short time. In future, we are considered to evaluate in a shorter time by shortening the irradiation time of light or examining other evaluation parameters or methods. It is expected to contribute to improve the realization of high efficiency of cultivation by optimizing light environment for cultivation in the plant factory using this technology. In the experiment of adding green light, it was confirmed that bioelectric potential response and CO2 consumption rate are different by green light ratio. It is generally considered that the green light is not important for plant growth compared to red and blue lights, but this result showed the activation of photosynthesis by green light. The reason for such a result is due to leaf structure of the plant. The surface side of leaf has a structure called palisade tissue in which cells are neatly arranged, and the irradiated light is easy to transmit. While the back side has called spongy tissue, and it is structured to easily scatter light.28 Each cell contains chlorophyll as a photosynthetic pigment, and it has a peak of light absorption around blue (450 nm) and red (660 nm).4 Among the irradiated light, the light close to the absorption spectrum of chlorophyll is absorbed by the palisade tissue on the surface side of the leaves. The light transmitted through the palisade tissue is scattered by the spongy tissue, and encounters the pigment then absorbed. Generally, when the pigments are unevenly distributed in the space, the light is less likely to be absorbed as compared with the case where the pigments are uniformly distributed. This phenomenon is called smoothing effect. The decrease in absorption due to the smoothing effect is remarkable for the wavelength that is well absorbed by the pigment. On the other hand, as the optical path becomes longer due to scattering, the opportunity for light to encounter the pigment increases, so that light is more likely to be absorbed. This is called pathway effect. The increase in absorption due to the pathway effect is remarkable for the wavelength which is hardly absorbed by the pigment. By these effects, the green light which is generally difficult to be absorbed by chlorophyll is also absorbed by the spongy tissue, and the light absorption rate of the leaf reaches 75 to 90%.29 Figure 12 shows a microscopic image of the cross section of sample plant Elan's leaf. In this image, the palisade and spongy tissues could be confirmed on the leaf surface and the back side. Thus, the CO2 consumption rate by the addition of the green light obtained in this study is due to leaf structure. It is thought that observed photosynthetic activity and the bioelectric potential responses for each variety of strawberry are influenced complicatedly by the internal structures of the leaves.
In this study, the responses of bioelectric potentials were compared for three varieties of strawberry when changing the wavelength ratio of irradiation light. When monochromatic light was irradiated on strawberries of Elan, Nyoho, and Akihime, it was found that the responses to each color of each variety differed. For Elan, the highest bioelectric potential response was observed when red-green light ratio is 70%: 30%, and the change in CO2 concentration with photosynthesis showed the same tendency. These results indicated that the plant biopotential measurement is a promising method to evaluate the spectrum of irradiation light which improve the photosynthetic activity in plant cultivation at low cost and in a short time. By using this technology, it is thought that not only energy efficiency in artificial cultivation environments such as plant factory can be enhanced but also unnecessary material cost can be saved, contributing to further development.
This work was supported by a JSPS KAKENHI Grant Number JP15KK0228.
Author declares that there is no conflicts of interest.

©2018 Murohashi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.