International Journal of
eISSN: 2573-2838


Research Article Volume 5 Issue 1
National Institute of Telecommunication - Inatel, Brazil
Correspondence: Filipe Loyola Lopes, National Institute of Telecommunication – Inatel. Av. João de Camargo, 510, Centro, 37540-000, Santa Rita do Sapucai, Minas Gerais, Brazil
Received: December 28, 2019 | Published: January 23, 2019
Citation: Lopes FL, Leite BT, Fraga AF, et al. Development of a portable Escherichia coli quantifier device. Int J Biosen Bioelectron. 2019;5(1):14?18. DOI: 10.15406/ijbsbe.2019.05.00145
Healthcare-associated infection is one of the main causes of mortality and healthcare costs increase. Microorganism’s identification and counting are essential for human health preservation. Escherichia coli is an important bacterium, which causes Healthcare-associated infection. Spectrophotometry method can be used to Escherichia coli counting, but the ordinary devices are not portable and are very expensive. The aim of this study is to develop a portable and low-cost device to quantifier the number of Escherichia coli ´s cells per mL based on Lambert-Beer law. Therefore, three steps were performed: device construction, software development and device testing. In the prototype test, a comparison was made between three measurement processes: concentration analysis results by visually counting cells; counting with a conventional spectrophotometer; and counting with a developed quantifier device. As conclusion, E. Coli quantifier device has some advantages: low cost, small size, it automatically performs 400 measurements of each sample to calculate the average and it is able to communicate instantly with a computer to facilitate interpretation of the results.
Keywords: count of microorganisms, Escherichia coli, analytical instrumentationATCC, American Type Culture Collection; COM, Serial communication computer portal; E. Coli, Escherichia coli; EMB Agar, Methyl Eosin Agar; Inatel, National Institute of Telecommunication; USB, Universal Serial Bus
Healthcare-associated infection is a serious public health problem, because it is responsible for raising the mortality rate and healthcare costs worldwide.1 Identifying contaminations caused by microorganisms, like Escherichia coli (E. Coli), is essential to preserve human health, but hospitals can be as reservoirs for viruses, fungi, bacteria and spores.2 Bacteria, in special, is part of the human flora and may pose health risks, especially for individuals with compromised clinical status, for example E. Coli can cause Nosocomial urinary tract infection.3–6 E. Coli is an anaerobic bacterium of bacillary morphology, Gram-Negative type. E. Coli is uropathogen in the fecal microbiota, which can colonize the urethral canal and ascend to the bladder, most frequent in women.3,7 The search for reduced time to microbiological analysis and technologies development results in new techniques to improve the analysis process and to minimize errors.8 Clinical analyzes using physicochemical techniques, such as spectrophotometry, have benefited immensely from quantitative microbiological analysis methods.9 Spectrophotometry has theoretical foundation in Lambert-Beer law and can be aid in medical decisions.10 Spectrophotometry is one of the most used qualitative and quantitative techniques due to the robustness and diversity of applications, however spectrophotometry typical devices is not portable and it is also expensive for applications in health care establishments with few financial resources.8–10 The cost of a simple spectrophotometer (model 225 D CELM-E) in Brazil was budgeted approximately 2,350.00 USD and its physical dimension is 385mm (width), 285mm (depth) and 215mm (height). The aim of this study is to construct and test the E. Coli quantifier device. This device has microcontroller (chip with embedded programming), it is portable, low-cost and it can quantify cells of E. Coli bacteria in samples with different concentrations of this microorganism.
The methodology of this research was divided into three stages: device construction, software development for computer communication and equipment testing.
Device construction
The development and construction of the device took place in the Medical Technologies Laboratory of the National Institute of Telecommunications (Inatel). The electronic boards were manually assembled and interconnected to the LauchPad MSP430F5529 (Texas Instruments) microcontroller platform (Figure 1). Power Source: Powered by USB standard (5 volts). LauchPad: Microcontroller platform (16-bit MSP430 25MHz), minimum hardware assembly for platform utilization and firmware development. Firmware development was done through layered programming using C language. Light Source: Choice of light source, infrared LED (IR LED) for monochrome beam production at 880nm and development of drive driver circuit. Sample: The holder mechanical development to receive the sample cuvette of solute to be measured. Transduction circuit: Circuit with photodiode, signal conditioning (amplification and filtering). Man-machine interface: liquid crystal display with two lines and 16 columns, keyboard development, hardware and firmware development for keyboard and display control. Communication with computer: Communication between the device and a computer through a USB cable.
Software development for computer communication
It was developed in the GUIDE platform of Matlab Program (MathWorks Inc), available in Inatel, it allows to perform mathematical operations and to use graphical tools for the manipulation of digital signals. The communication between the computer and the equipment was performed through serial protocol with USB cable. To this end, it was necessary to divide the information into eight-bit packets. These packages are reassembled by the software when they arrive at the computer through the virtual COM port. The software objective is to establish instant communication with the E. Coli quantifier through USB cable in order to capture the data read from biological samples in order to facilitate the interpretation of the user through graphs and values. The software also performs simple linear regression of the sampled points, aiming to calculate the coefficient of determination (R²) of the curve plotted from different concentrations of microorganisms.
Equipment testing
The test was performed by comparing the results between the developed device measurements, the spectrophotometer measurements CELM-E model 225 D provided by Inatel, and visual counting of bacteria colonies. In order to achieve the E. Coli concentration measurements, the samples were artificially contaminated (saline solution) from an American Type Culture Collection (ATCC) strain of E. Coli 25922, provided by SANOBIOL (Brazilian Industry). The culture was performed in a selective medium for enterobacteria, Methyl Eosin Agar (EMB Agar), and the logarithmic growth phase was used. The suspension of the microorganism was obtained at the concentration of 9mL of saline solution, with turbidity corresponding to 1x106(cell/mL), according to the Macfarland scale.9 For each treatment, tubes with 5mL of bacteria suspension were tested. Thereafter, each treatment was spread on the surface of different EMB agar plates through Drigalsk. The colonies grew for 48 hours at 35°C for three counting methods (Figure 2). Visual counting of the cell concentration (cells/mL) in the samples was performed through the image obtained by digital photography of the cultivated Petri dishes. The pen tool of the Paint program (Microsoft) was used for the counting. The analysis of the results was obtained using Matlab and Excel (Microsoft), using simple linear regression and coefficient of determination (R²). The resulting data was analyzed separately, but always between two distinct groups.
The prototype was developed using low-cost and easily accessible components. Material price of prototype is 64.48 USD and this physical dimension is 107mm (width), 205mm (depth) and 40mm (height) (Table 1) (Figure 3). The device can be calibrated. User can calibrate opaque sample (0% transmittance) and transparent sample (100% transmittance distilled water or saline solution). The results can be graphically viewed on a computer screen (Figure 4). Developed program can check the stability of the readings performed, since the equipment was programmed to perform 400 repetitions of sample reading and then plot on a graph that must be constant, minimizing error margins. The software is able to mark "n" read points as the command is given by the user. With marked "n" points, the software offers the option of performing simple linear regression and calculation of the coefficient of determination (R²) with just the click of a button. Visual counting of the cell concentration (cells/mL) in the samples was performed through the image obtained by digital photography of the cultivated Petri dishes. The results of visual counting can be found in Table 2 and an image of sample can be seen in Figure 5. Data obtained commercial spectrophotometer and device developed can be seen in the Table 3. The comparison between the data obtained from conventional spectrophotometer and visual counting result, as shown in Figure 6A & Figure 6B, demonstrates the comparison between E. Coli counter and visual counting. From the graphs it was possible to apply the simple linear regression method and the calculation of the coefficient of determination (R²).
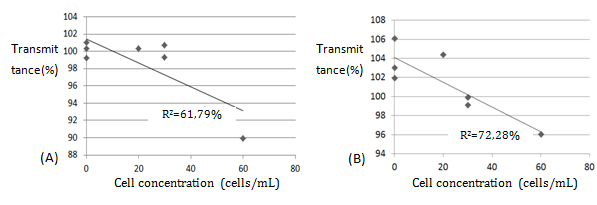
Figure 6 (A) Comparison between visual method count and conventional spectrophotometer. (B) Comparison between visual method and E. Coli counter.
Description |
Quantity |
Unit price (USD) |
Total price (USD) |
MSP430F5529 LaunchPad Development Kit |
01 |
20.78 |
20.78 |
Photodiode 880nm + import taxes |
01 |
2.56 |
2.56 |
Infrared LED + import taxes |
01 |
3.02 |
3.02 |
Display 16x2 |
01 |
8.62 |
8.62 |
Op. Amp. LM741 |
01 |
0.52 |
0.52 |
Plastic Box |
01 |
13.46 |
13.46 |
Sample support |
01 |
13.59 |
13.59 |
Printed circuit board |
01 |
1.93 |
1.93 |
Total |
64.48 |
||
Table 1 Cost of components in Brazil to assembly of the prototype. The values are in US Dollars (USD) with a price of 01.00 USD equal to 3.68 Brazilian Reais. In this analysis the labor costs was not considered
Escala Macfarland |
Contagem visual |
100 |
60 |
10-1 |
30 |
10-2 |
20 |
10-3 |
30 |
10-4 – 10-6 |
nulo |
Table 2 Result of the visual count
Macfarland scale (Dilution) |
commercial spectrophotometer |
device developed |
Opaque sample |
0% |
0% |
100 |
89.9% |
96.0519% |
10-1 |
99.9% |
99.0967% |
10-2 |
99.9% |
102.0102% |
10-3 |
100.7% |
99.9124% |
10-4 |
104.7% |
106.098% |
10-5 |
99.2% |
103.033% |
10-6 |
98% |
101.94% |
Transparent sample |
100% |
100% |
Table 3 Comparison of measure with commercial spectrophotometer and E. Coli counter device, using Macfarland scale for dilution
Identifying and controlling bacterial colonies in a hospital is very important to decrease mortality rate and healthcare costs.1 Analysis and the study of cases of microbiology using techniques such as spectrophotometry are extremely important.8,9 Low cost and portable equipment are very relevant for using in bio safety hospitals, food industries and other environments subject to E. Coli contamination. The spectrophotometer used as reference was budgeted approximately 2,350.00USD. The cost of the parts for the assembly of the prototype was 64.48USD. This comparison is not complete, since they do not constitute labor costs for prototype development or the cost of product certification. However, it may be a sign of price improvement. An important factor influencing the low cost of the prototype is that E. Coli quantifier uses specific wavelengths, minimizing the use of components for different wavelengths. In contrast, the commercial spectrophotometer has many applications and uses many wavelengths.10 It is important to note that the equipment developed (107mm width, 205mm depth and 40mm height) was much smaller than commercial equipment (385mm width, 285mm depth and 215mm height).In the comparison between the data obtained from conventional spectrophotometer and E. Coli counter, the E. Coli quantifier device shows greater correlation (72.28%) with visual measurement than conventional spectrophotometer (61.79%), since the coefficient of determination (R²) was higher, although in some points it presented less accuracy. It is important to note that the measurements were performed using a low concentration of microorganisms, which reveals a high sensitivity of both equipment tested and that the errors of accuracy presented by the prototype can be reduced for higher concentrations. Both methods showed a strong correlation between dependent and independent variables (higher than 60%). According to Pontes10 perfect correlation (R²=100); very strong correlation (90<R²<99); strong correlation (60<R²<90); mean correlation (30<R²<60); poor correlation (10< R²<30); and null correlation (R²<10).9 For future, it would be interesting new tests with higher concentrations of cells, using other wavelengths, other types of microorganisms and improvement of the firmware for the reduction of accuracy errors.
An E. Coli counter prototype was developed and underwent initial tests to compare measurements performed by the developed device and a conventional spectrophotometer. Both results were compared with the visual method, resulting in coefficient of determination of 72% and 62%, respectively. The developed device has the advantages: higher correlation to visual method, low cost, reduced size (portable), 400 readings of each sample for calculation of average in order to reduce errors and communicates automatically with computer to facilitate biostatistician analysis. The device has the potential to assist the analysis of microorganisms in hospitals, clinics and companies.11
We thank Inatel for the use of laboratories, components and materials and a team of researchers.
Author declares that there is no conflict of interest.

©2019 Lopes, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.