International Journal of
eISSN: 2573-2838


Review Article Volume 5 Issue 6
Department of Biomedical Engineering, Sheikhbahaee University, Iran
Correspondence: Hamidreza Shirzadfar, Department of Biomedical Engineering, Sheikhbahaee University, Isfahan, Iran
Received: September 15, 2019 | Published: November 4, 2019
Citation: Shirzadfar H, Dohani S, Ghaedi M, et al. Creating the new generation coils to generate a uniform magnetic field using for medical applications: simulation and analysis. Int J Biosen Bioelectro. 2019;5(6):179-183. DOI: 10.15406/ijbsbe.2019.05.00174
The magnetic coil was invented in the mid-19th century by Herman von Helmholtz. This coil can create a uniform magnetic field. The main structure of the coil consists of two identical coils that are in the direction of one of the three-axis X, Y or Z. Around each coil, there is a series of conductors with the same current I. The best performance of this coil is when the distance between the coils is equal to the radius of the coil.1,2 As the current flows through the wires, a magnetic field is created around the coil and a homogeneous magnetic field is formed inside the coil (Figure 1).3,4 This coil is used for medical applications such as hyperthermia and smart drug delivery. In hyperthermia for the treatment of cancer cells increased tissue temperature because they are sensitive to temperature.5 In this method, directly or through the intravenous system, the fluid containing the magnetic nanoparticles enters the target tissue, then the coil produces an alternating magnetic field.6 This magnetic field causes the nanoparticles to emit heat. When the tissue temperature reaches above 42degrees cancer cells are destroyed.7 The iron oxide nanoparticles are used for drug delivery.8 The size of magnetic particles is about 5-50nm.9 When the nanoparticles combine with an external field, they allow the particles to be delivered to the desired region.10,11 Smart drug delivery can eliminate side effects and reduce the dose of medication needed.12,13 The purpose of this article is to present a new model of coils for medical applications.
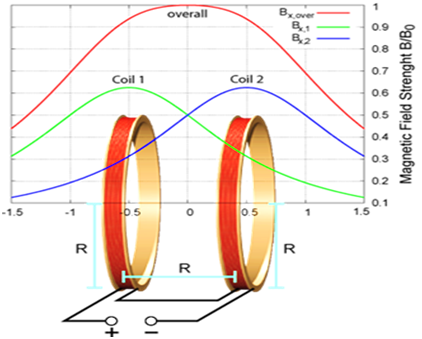
Figure 1 The schematic of the Helmholtz coil and the magnetic field curves.4
Keywords: Helmholtz, homogeneous, axis, hyperthermia, drug delivery, magnetic field flux, three-dimensional
In this investigation, the simulation models are used by the software COMSOL. This software has many applications in the fields of electrical engineering, mechanics, chemistry, physics and more. This software can communicate with other engineering software such as MATLAB, Excel and etc. It can also be installed on 32-bit and 64-bit windows. COMSOL was created in 1986 by two students at the Royal Swedish Institute of Technology named Farhad Saeedi and Svante Litmark.14 The software can perform three steps pre-processing, processing and post-processing. There are many modules in COMSOL for a variety of applications such as electrical, chemical and mechanical applications.15,16 The Helmholtz Coil is a device for creating a uniform magnetic field within a range.17 A Helmholtz consists of two identical circular magnetic coils whose distance is equal to the radius of the coils (Figure 2). As the current passes through the coils, a uniform magnetic field is created in a three-dimensional region of space inside the coils (Figure 2A & 2B). The magnetic field in the Helmholtz coil can be generated by both alternating current (AC) and direct current (DC). Most coils use direct current to generate static magnetic fields, but many applications as magnetic field sensitivity testing, scientific testing, and biomedical studies require different magnetic fields. In some other applications, the coil is used to destroy the Earth's magnetic field. This coil creates an area whose magnetic field intensity is close to zero.18
As we know, cylindrical coils have some limitations for medical investigations like in drug delivery, study the behavior of the magnetic particles19‒22 and the in the limit size of the coil in MRI. In this project, we tried to present the new models of the magnetic coil using the different geometries to achieve the appropriate model for using in biomedical applications particularly in the weak range of magnetic field. After study the several shapes of the coil, we concluded that the trapezoidal shape is more valuable as the form and the uniform magnetic field curve form other models. In following, we present the models and obtained the result.
First model
In this simulation, three circular coils with a radius of 10cm are used, arranged in a triangle, with the angles of the coils present at two vertices 60 and -60degrees. As we can see in the magnetic flux density graph (Figure 3B) it is homogeneous along the x and y axes, but along the z-axis we have instantaneous jump diagrams, we can only increase the number of coil windings that exist at the base of the triangle. Also, eliminate the oscillations in the z-axis. This simulation was not used because the angle between the coils had to be precisely executed otherwise the result would not be optimal and this would be difficult. The simulated magnetic flux in the three-dimensional space between the coils is also shown in Figure 3A.
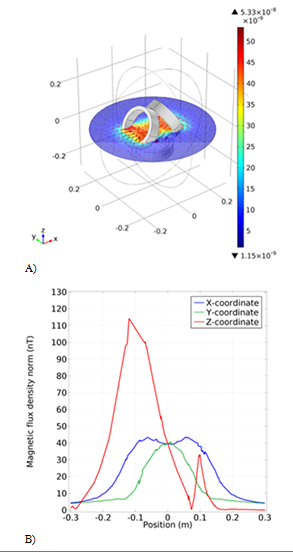
Figure 3 The first simulation model of the coil. A) The schematic of the coil with the magnetic field arrow. B) The Magnetic flux density results from the new coil in different coordinates.
Second model
In the next simulation we used six circular coils with a radius of 5cm arranged at angles 51, -51, 50, -50 and zero degrees. As we can see in the magnetic flux diagram, we are homogeneous along the x-axis, but there is a small range along the z-axis in the center of the coil where we need the maximum magnetic flux, as shown in Figure 4B. It is possible to solve these problems with the homogeneity of the flux with a slight variation in the number of coils of coil winding, but we also omitted this model due to the difficulty of precise angles between the coils. The simulated magnetic flux in the three-dimensional space between the coils is also shown in Figure 4A.
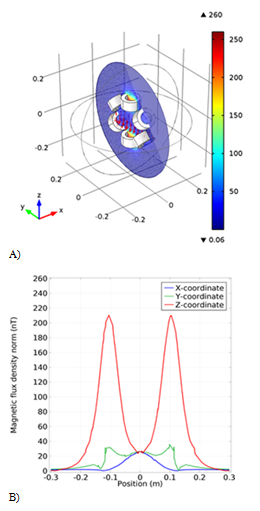
Figure 4 The second model simulation of the coil. A) The schematic of the coil with the magnetic field arrow. B) The Magnetic flux density result from the new coil in different coordinates.
Third model
The next design consists of three circular coils with a radius of 10cm, 16cm and 21cm, with two outer coils having a 90degree angle. The magnetic flux is homogeneous along the x-axis, but it is highly oscillatory along the y and z axes, and especially along the z-axis (Figure 5B), so the scheme was not used. The simulated magnetic flux in the three-dimensional space between the coils is also shown in Figure 5A.

Figure 5 The third model simulation of the coil. A) The schematic of the coil with the magnetic field arrow. B) The Magnetic flux density result from the new coil in different coordinates.
Forth model
In the next simulation, three triangular coils with a base of 40cm and a height of 35cm are 12cm apart. The magnetic flux is homogeneous along the y-axis as shown in (Figure 6B), indicating that the flux is higher in the center, but in the other two directions, the magnetic flux is not homogeneous and there are many oscillations. The simulated magnetic flux in the three-dimensional space between the coils is also shown in Figure 6A.
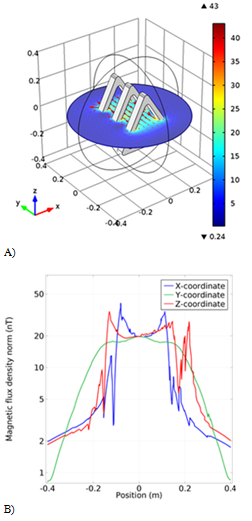
Figure 6 The fourth model simulation of the coil. A) The schematic of the coil with the magnetic field arrow. B) The Magnetic flux density result from the new coil in different coordinates.
Final model
In the final simulation of the two coils, a trapezoid with a large base of 30cm, a small base of 15cm and a height of 15cm is used. Each coil has 40 reel coils. As shown in Figure 7B), the coordinates of the magnetic flux are homogeneous along all three axes. The simulated magnetic flux in the three-dimensional space between the coils is also shown in Figure 7A.
The main purpose of this project is to design a Coil magnetic coil model. We designed the various models using the COMSOL Multiphysics simulation software which mostly applies the finite element method. Different coil with dissimilar schematics was simulated. The goal was to design a magnetic coil with specific features and more suitable for producing a uniform magnetic field varying from 0 to 100nT. Smart drug delivery plays a significant role in the treatment of cancers. With using nanotechnology knowledge, the drug works only in the affected area and does not cause complications in healthy areas of the body. Furthermore, this project aims to design a new magnetic coil that differs from the usual circular coils used in medical applications. According to its geometric shape, the trapezoidal coil which designed has the patient-facing more open space. The freedom of technician or physician practice is helpful and the patient will feel more comfortable. These properties and the geometrical shape of the trapezoidal coil have not eliminated the homogeneous property of the field. Finally, the last model was considered as a selected model because the field was uniform in all three axes compared to the other models. Also, due to its appearance, this model reduced the functional limitations. The field distortion was negligible and it was also easier and more feasible to build compared to the other coils. As a final point, we have fabricated the trapezoidal coil with an intelligent system that permits us to measure the magnetic field inside the coil in real-time and we would like to present it in an upcoming article.
None.
None.
Authors declare that there is no conflict of interest.

©2019 Shirzadfar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.