International Journal of
eISSN: 2573-2838


Mini Review Volume 1 Issue 1
Tissue Engineering and Biomicrofluidics Laboratory, Indian Institute of Technology, India
Correspondence: Sanjeev Kumar Mahto, Tissue Engineering and Biomicrofluidics Laboratory, School of Biomedical Engineering, Indian Institute of Technology, Banaras Hindu University, Varanasi, 221005, Uttar Pradesh, India, Tel +91-7617052884
Received: August 09, 2016 | Published: September 30, 2016
Citation: Anjali, Poddar S, Mahto SK. Revisiting pulmonary diseases using microfluidic technology. Int J Biosen Bioelectron. 2016;1(1):1–5. DOI: 10.15406/ijbsbe.2016.01.00001
Pulmonary diseases such as chronic obstructive pulmonary disease, asthma and tuberculosis are among the leading causes of death globally, accounting for nearly 4 million of all deaths worldwide every year. Prevention, management and treatment of these deadly diseases require early detection of pathogens and accurate study of pathophysiology of the respiratory system. In recent years, microfluidic technology has emerged as a powerful tool for recapitulation of the physiological and pathological scenarios of the lung for enhancing the current understanding of pulmonary disorders. This review paper outlines the advantages of microfluidic devices in providing improved insights into the mechanism of the pulmonary diseases, identification of their novel biomarkers and the development of new and advanced therapeutic strategies. Furthermore, several microfluidic lung models are discussed along with progresses made from the aboriginal to till date.
Keywords: pulmonary diseases, pathophysiology, microfluidic devices, biomarkers, therapeutic strategies
POCD, point-of-care device; COPD, chronic obstructive pulmonary disease; ARDS, acute respiratory distress syndrome; WHO, world health organization; TB, tuberculosis; BlaC, β lactamase; CF: Cystic Fibrosis; CFTR: Cystic Fibrosis Trans membrane conductance Regulator; DNA: deoxyribonucleic acid; Mtb, mycobacterium tuberculosis; 3D, three-dimensional; PDMS, polydimethylsiloxane; PCR, polymerase chain reaction
The human respiratory system consists of nose, pharynx (throat), larynx (voice box), trachea (windpipe), bronchi, and the lungs.1 The nose, pharynx, larynx, trachea and bronchi together form the respiratory airways. The bronchus continues to divide within lung into many narrower and shorter airways known as bronchioles.2 The branching networks of pulmonary airways are lined with a viscous liquid film secreted by airway epithelial cells.3 Terminal bronchioles are clustered in grapelike sacs, the alveoli. Gaseous exchange between air and blood takes place in the alveoli. The lungs contain approximately 500 million alveoli, each about 200 to 300 μm in diameter. The alveolar wall consists of a single layer of type I alveolar cells. Each alveolus is surrounded by a network of pulmonary capillaries. The interstitial space between pulmonary capillaries and alveolus forms a very thin barrier which facilitates gaseous exchange. Apart from type I alveolar cells, 5 % of the alveolar surface epithelium is covered by type II alveolar cells, which secrete a phosphor lipoprotein complex known as pulmonary surfactant that facilitates lung expansion.2 In addition to type II alveolar cells, surfactant proteins are also produced by Clara cells of peripheral airways. The pulmonary surfactant produced from these cells is absorbed into the luminal air-liquid interface of the pulmonary airways and thus reduces the surface tension to values as low as zero during expiration. This in turn prevents the airway closure.4 Lung epithelium is a highly complex tissue where epithelial cells, sub epithelial fibroblasts and the extracellular matrix of the airway wall are intricately involved in maintaining and regulating the structure and function of the lung.5
Lung diseases
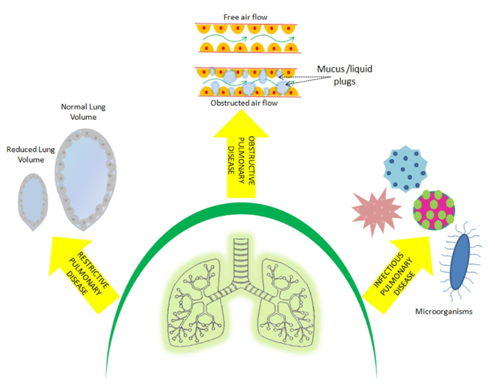
Respiratory diseases and disorders encompass pathological conditions that mostly affect the organs and tissues that take part in gaseous exchange (Figure 1). Notably, such abnormalities affect the airways, the physiology structure of lung tissue, and the pulmonary circulation.6 The lung diseases are broadly classified into obstructive, restrictive and infectious diseases (Table 1).
Type of Diseases |
Respiratory Diseases |
Conventional Diagnostic Methods |
Obstructive Diseases |
Chronic Obstructive Pulmonary Disorder (COPD) |
Spirometry, Cell migration assays including trans-well assay and the under-agarose assay |
Asthma |
Patient’s medical history, spirometry test, physical examinations |
|
Acute Respiratory Distress Syndrome (ARDS) |
Gene expression analysis. |
|
Restrictive Diseases |
Cystic Fibrosis |
Flow-cell based system. |
Infectious Diseases |
Tuberculosis |
Acid-fast smear using sputum, interferon gamma release assay. |
Table 1 Classification of the respiratory diseases and their conventional diagnostic methods
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the world. The COPD burden is projected to increase globally in the coming decades because of continued exposure to COPD risk factors and aging of the population.7 COPD, a chronic inflammatory disease, is generally an adult age problem8 and characterized by breathing difficulty as a consequence of narrowed airways. Long term exposure of lungs to noxious particles or gases, such as cigarette smoke, is considered as the main cause of COPD. According to WHO, COPD will become third leading cause of death by 2030.9
Similarly, asthma is also amongst the chronic inflammatory disorders characterized by active hyper responsiveness to a variety of stimuli e.g. airborne allergens. It results from a complex interaction among inflammatory cells, mediators and airways.8 According to the most recent revised global estimate of asthma, it is found that as many as 334 million people have asthma, and that the burden of disability is high.10
In acute respiratory distress syndrome (ARDS), the integrity of the alveolar-capillary barrier is compromised due to fatal events such as severe chest trauma, serious infection in the blood or other tissues, and bacterial or viral insult to the lung. Consequently, a proteinaceous fluid leaks from capillaries into the interstitium and floods alveoli. Furthermore, inflammatory cells, neutrophils, and macrophages infiltrate alveoli and airways. The presence of fluid and inflammatory mediators alters concentration and composition of the lung surfactant and accounts for surfactant malfunction in ARDS.11
Tuberculosis (TB) is an infectious disease, caused by Mycobacterium tuberculosis. It is one of the most deadly diseases and that kills over one million people each year and infects one–third of the world’s population.12 The WHO’s goal to eradicate tuberculosis by 2050 is not still attainable due to limitations of current technologies for diagnosis, treatment and prevention. The WHO had estimated to have more than 2 million new cases of tuberculosis between 2011 and 2015.13
Cystic fibrosis (CF) patients suffer from a defect in the cystic fibrosis trans membrane conductance regulator (CFTR) gene. As a consequence, the mucus layer in the conductive airways becomes very viscous and the mucociliary clearance mechanisms are highly impaired. This results in frequent and recurrent infections of the CF airways, with risk of pneumonia.14
Conventional study models and diagnostic methods
Timely and effective management and control of many pulmonary diseases remain suffered from the lack of proper preventive care and treatment till date. Despite intensive research efforts, the mechanism of the pulmonary diseases remains poorly understood, mainly due to lack of suitable in vitro models that recapitulate the complex in vivo conditions accurately.
Conventional assay methods used for diagnosis of the pulmonary disease primarily include spirometry, cell migration assay,9 acid fast smear using sputum,12 gene expression analysis,15 cell culture assays,16-18 interferon gamma release assay.18 In addition, for understanding the mechanism of pulmonary diseases, researchers have conducted their studies conventionally in human patients,9 animal models4,14 and using in vitro lung tissue slices.16 The specific limitations of the conventional study models as well as of the conventional diagnostic methods are summarized in (Table 2) (Table 3), respectively.
In Vitro Study Models |
Limitations |
Animal Models and Human Patients |
Small airways in orders of millimetres cannot be adequately studied in animal models and human patients,9 expensive and raises ethical concerns. Animal models do not show immunological responses similar to the level of human.14 |
In-VitroLung Tissue Slice Culture |
Human organ slices can be difficult to obtain and the survival of slices is limited to a few days.16 |
Table 2 Limitations of the conventional study models used for diagnosis of the lung diseases
Conventional Diagnostic Methods |
Shortcomings |
Spirometry |
Requires patient cooperation that can be difficult for children or patients with severe condition.9 |
Cell Migration Assay |
Unable to control gradient . |
Acid Fast Smear Using Sputum |
Takes few months to become positive with sensitivity of 20-80%.12 |
Gene Expression Analysis |
Laborious, samples are subjected to considerable ex-vivo perturbation resulting in documented phenotypic and functional changes.15 |
Cell Culture Assay |
Unable to mimic the in vivo conditions,16, 17 contamination is a big challenge.17 |
Interferon Gamma Release Assay |
Unable to count the number of CD4 cells secreting the cytokine, that makes them less accurate.18 |
Table 3 Conventional diagnostic methods and their shortcomings
Opportunities for microfluidic technologies to explore pulmonary diseases
Developing a physiologically and anatomically accurate model of the pulmonary system mimicking both the cellular structure and physiological function of the lung is among the daunting challenges of modern respiratory pathophysiology. Over the past few decades, several research groups have made sincere efforts to design and develop biomimetic microfluidic systems closer to the physiological scenarios of the lung structure and their function for highlighting the molecular processes underlying pathophysiology of the respiratory diseases (Table 4).6 Microfluidic systems have been used widely as platforms for the culture of mammalian cells to study various physiologic and pathologic conditions in vitro. The ability of microfluidics to recapitulate the in-vivo condition has made it popular among researchers.11 This advanced technology provides excellent platforms for identifying novels biomarkers, developing new insight into mechanisms of the disease, testing new drug candidates. Moreover, lung inflammatory diseases can be studied over several weeks in such micro-devices incorporated with cells harvested from both normal and diseased persons.9
Type of Diseases |
Respiratory Diseases |
Scenario Mimicked |
Technique Employed |
Obstructive diseases |
Chronic obstructive pulmonary disorder (COPD) |
Neutrophil infiltration into airways through chemo tactic migration |
The pressure difference between the inlet and outlet wells establish gradient flow of the chemo attractant 26 |
Asthma |
Detection of asthma based on cellular function |
Detection of asthma based on cellular function |
|
Acute respiratory distress syndrome (ARDS) |
Liquid plug formation |
Air tank, airflow meter, valves, syringe pumps, and a computer is integrated to the microfluidic platform 21 |
|
Repeated opening and closure of airways |
Automated fraction controller integrated with the microfluidic device 22 |
||
Restrictive diseases |
Cystic fibrosis |
Mucus plug in CF bronchi |
Cell culture chamber separated by a membrane, and a thick mucus layer simulated by using alginate hydrogel 23 |
Oxygen tension in CF airways |
Compartmentalized platform shows connections between oxygen rich and oxygen depleted regions 14 |
||
Infectious diseases |
Tuberculosis |
Development of a diagnostic system |
Paper based microfluidic using salt-induced gold nanoparticle (AuNP) 25 |
Detection of a reporter enzyme BlaC |
A circular microfluidic device for capturing cytokine secreting CD4 cells using glutaraldehyde 12 |
Table 4 Summary of opportunities for microfluidic technology to explore pulmonary diseases
A variety of pulmonary diseases such as COPD, asthma, ARDS are profoundly associated with the surfactant dysfunction that leads to liquid plug formation across the airway lumen.19 Several animal model studies have shown during such lung disorders severe tissue-level damage to the distal lung airways due to repeated closure and reopening process 4. To mimic exactly the in vivo conditions, Huh et al.20 developed a compartmentalized microfluidic airway models and demonstrated that the reopening of occluded microfluidic airway causes severe injury of pulmonary epithelial cells.20 In the lung airways, rupturing of the liquid plugs leads to abnormal breath sounds known as crackles. To simulate this scenario, a three-dimensional (3D) microfluidic device was developed to detect acoustically the crackling sound and it was demonstrated that there is a higher risk of cell injury when liquid plugs become very thin. They demonstrated cellular level of lung injury under flow condition using this device.19 To create on-chip liquid plugs, various components including an air tank, airflow meter, valves, syringe pumps, and a computer are integrated to the microfluidic platform.21 Surfactant is known to reduce the surface tension during expiration to stabilize the liquid present in the airway. To investigate the physiological role of surfactant, a microfluidic model of small airway of the peripheral lung was recently developed and it was shown that addition of a physiological concentration of surfactant to the propagating liquid plug protects epithelium and reduces cell death significantly.4 Despite much intensive research using the conventional models, the responses of airway barrier to environmental agents remained poorly understood. Towards understanding such airway behaviors Blume et al.22 recently developed a novel dynamic 3D in vitro airway epithelium model in which they integrated the microfluidic culture system with automated fraction collector that allows more sensitive early phase analysis of barrier responses to environmental impacts.22
Similarly, more advanced platforms of models airways specific to cystic fibrosis (CF) have recently surfaced. Traditionally, flow-cell based system has been used for studying the mechanism of cystic fibrosis. However, such system lacks the in-vivo environment of the lung airways. Skolimowski M et al.23 developed a microfluidic model of CF bronchi. The model consists of 2 cell culture chambers separated by a membrane. The thick mucus layer spotted in the CF patients was simulated by an alginate hydrogel above the membrane and the epithelial cells were cultured in the bottom chamber. They inoculated Pseudomonas aeruginosa PAO1 strain to the hydrogel layer for studying the antibiotic treatment on bacterial infection.23 Also, the same groups designed a modular microfluidic airway model that simulates the oxygen tension in different compartments of the CF airway. This model permits to freely reconfigure connections between oxygen rich and oxygen depleted regions. It was able to mimic different scenarios such as clogging of the ostia in recurrent sinusitis or the development of mucus plug in the bronchioles that was previously not possible using a flow-cell based system.14
For an effective and efficient pathogen detection method, assays should ideally be cost-effective, fast, sensitive, and accurate. A microfluidic device has the ability to improve the performance of assays by facilitating less consumption of reagents, rapid analysis, high reliability and sensitivity as well as integrating multiple processes in a single device.24 Therefore, microfluidic technology has also been exploited to design and develop various types of micro-devices for the diagnosis of tuberculosis (TB), pneumonia and asthma.
Treatment of TB essentially requires effective detection of infectious pathogen.24 Tsai et al.25 presented a microfluidic paper based diagnostic device that utilized salt-induced gold nanoparticle (AuNP) based colorimetric diagnosis of TB DNA sequences. This device has shown the potential to facilitate high-throughput and high-content screening and help avoid the requirement of sophisticated optical based detection equipment as well as a well-trained technician.25 At present, the biggest challenge with the treatment of TB is its prompt diagnosis. WHO estimated tuberculosis detection rate to be just 63%.26 For this purpose, Rosenfeld et al.12 developed a novel microfluidic device for rapid, sensitive, specific and quantitative detection of a reporter enzyme, BlaC specifically produced from Mycobacterium tuberculosis (Mtb).12 Similarly, a circular microfluidic device was designed and presented for the accurate diagnosis of Mtb by capturing the cytokine secreting CD4 cells using glutaraldehyde.18
Asthma is very difficult to accurately diagnose because its symptoms can be transient and common tests are susceptible to user error. For accurate diagnosis of asthma, a microfluidic device was recently developed that provides an alternative platform for the clinicians to characterize asthma based on the cellular function. They showed that neutrophils move slower than normal in response to inflammation among asthmatics compared to those with allergic rhinitis.26 Also, COPD correlates well to neutrophil infiltration into airways through chemo tactic migration. Based on the same physiological phenomena, a microfluidic platform was developed for evaluating neutrophil chemotaxis to sputum samples taken from a COPD patient. This assay furthermore revealed an increased level of neutrophil chemotaxis as observed physiologically in COPD patients.9
The cone-shaped, sponge-like organs, lungs, represent an extraordinary example of natural air filter for facilitating oxygen supply to the human body. To redesign or reproduce them either partly or to the full capacity, achieving the physiological-level architecture and complexities is an important issue and therefore considered one of the most challenging tasks. Several research groups have dared to confront the situation with the help of microfluidic technology. Undoubtedly, some of the best innovations including a small (merely few centimeters in size) flexible chip mimicking the alveolar level intricacies have come up in the past few years. Such devices are aimed to replenish the need of those who suffer from compromised breathing because of severe pulmonary diseases. This technology thus facilitates gaining an insight into the pathophysiology of the lung complications and in turn possibly help improve the therapeutic approaches while dealing with potentially fatal pulmonary diseases.
Although microfluidics is explored as an alternative platform for culturing cells in vitro, it has its own limitations that one cannot overlook while performing cell culture studies. Considering its advantages of design flexibility, lesser amount of laboratory reagents consumption, real-time analysis in spatial and temporal resolution, the typical challenges associated with these devices cannot be ignored. A major hurdle that accompanies every microfluidic device is the hydrophobicity of its polymeric material e.g., polydimethylsiloxane (PDMS). Due to its inherent hydrophobic nature, surface treatment of PDMS devices becomes an important and necessary step to mimic the hydrophilic physiological environment for mammalian cell culture. This can be overcome by exposing the culture platform with plasma rays that render the surface of PDMS hydrophilic.27 Secondly, culture protocols need to be optimized at every step for microfluidic-specific conditions; as long as minute volumes of reagents are involved as it needs patience and careful handling. Creating a dynamic system inside the microfluidic device ask for some additional components such as syringe pumps and its accessories that require expertise and dedication to gain full operational control.28 Recreation of artificial structures with physiological-level resemblance demands microarchitectured chips. Designing of these complex microarchitectures can get better with some professional softwares specifically dedicated for this purpose. In spite of being stymied by such barriers, microfluidics still stands as one of the most exploited technologies for conducting experiments that imitates physiological-level and disease-specific microenvironments.
The microfluidic technology has been exploited to create numerous excellent platforms that recapitulate the in vivo conditions of the lung environment very closely and therefore facilitate better insight of the physiological and pathological conditions of the lungs. Such innovations ultimately have enhanced the current understanding of pulmonary disorders and thus may help establishing improved guidelines for developing therapeutic interventions. Nevertheless, there remains a critical need to design and develop disease-specific point-of-care devices (POCDs) using microfluidic technology for the early, effective and onsite diagnosis of pulmonary diseases.
The author declares no conflict of interest.

©2016 Anjali,, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.