eISSN: 2471-0016


Mini Review Volume 1 Issue 3
1Department of Pathology, Leiden University Medical Center, Netherlands
1Department of Pathology, Leiden University Medical Center, Netherlands
Correspondence: Celine Klessens QF, Department of Pathology, Leiden University Medical Center, L1Q Room P0-107, P.O. Box 9600, 2300 RC Leiden the Netherland, Tel 31(0)71-526 5008, Fax +31 (0)71-526 6952
Received: January 01, 1971 | Published: September 28, 2015
Citation: Celine KQF, Hans BJ, Emile H. Tissue storage of dipeptides as protein guards against oxidative injury in patients with type 2 diabetes and its micro vascular complications. Int Clin Pathol J. 2015;1(3):46–49. DOI: 10.15406/icpjl.2015.01.00011
Histidine-containing dipeptides such as Carnosine (β-alanine-L-histidine) and anserine (β-alanine-L-methyl histidine) are not incorporated into proteins; instead, they are stored in peripheral skeletal muscle and other organs, including the kidney, retina, and myocardium. Carnosine has several protective functions in health and disease state. This mini review provides a current overview of the knowledge of the histidine containing dipeptides in disease accompanies by oxidative stress like diabetes and diabetic micro vascular complications. It also highlights the performed experimental studies of histidine-containing dipeptides in relation to tissue damage related to (non-invasive) human studies. Finally, it describes future perspectives of the therapeutic use of histidine-containing dipeptides in health and disease.
Keywords: histidine-containing dipeptides, oxidative stress/injury, carnosine, carnosinase, diabetes, diabetic micro vascular complications
Type 2 diabetes mellitus affects more than 400million patients worldwide1. The disturbed glucose metabolism in this metabolic disorder results in increased production of reactive oxygen species (ROS), and in oxidative systemic damage, accompanied by reduced defense and repair mechanisms against oxidative injury.2–5 In addition the development of micro vascular complications of diabetes in a subset of patients (30-50 %)6 such as diabetic nephropathy, retinopathy and cardiomyopathy have been associated with excessive oxidative stress. In several studies oxidative injury has been implicated as a driving force in micro vascular complications.7–9 Since oxidative stress, carbonyl stress, and transforming growth factor (TGF)-beta hyperactivity are prominent features in patients with type 2diabetes, new treatment modalities should be focused on natural repair mechanisms that are beneficial to diabetic patients.
Histidine-containing dipeptides such as Carnosine (β-alanine-L-histidine) and anserine (β-alanine-L-methyl histidine) are stored in high concentrations in various tissues. These dipeptides are not incorporated into proteins; instead they are stored in peripheral skeletal muscle cells and other organs, including the kidney, pancreas, retina, and myocardium. β-alanine is the rate limiting amino acid in the biosynthesis of these histidine-containing peptides, but can be provided by oral intake. β-Alanine is internalized by cells via the taurine transporter in order to be synthesized into Carnosine or anserine for subsequent intracellular storage.10,11 Carnosine is synthesized by the enzyme Carnosine synthase (CARNS).12 The gene that encodes CARNS is ATPGD1,13 the expression and distribution of CARNS and ATPGD1 require additional studies.14 In primates, carnosine is degraded predominantly by the enzyme carnosinase-1 (CNDP1), which is synthesized and secreted by the liver into the circulation. CNDP1 is encoded by the CNDP1 gene (Figure 1).15 In rodents, CNDP1 is absent in the circulation. Two forms of Carnosinase are expressed in primates: carnosinase-1, which is also called serum Carnosinase, and carnosinase-2, which is also called tissue Carnosinase or cytosolic nonspecific dipeptides.16
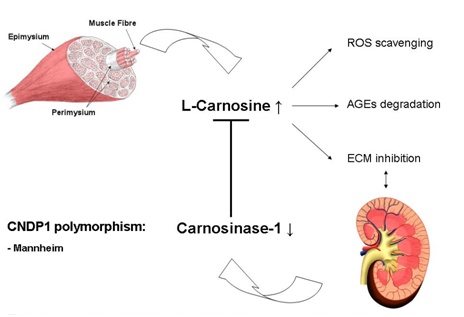
The highest concentration of carnosine can be found in the skeletal muscle (7.2-30.7mmol/kg dry muscle weight). Carnosine are secreted into the circulation by skeletal muscle cells during physical exercise, which is in line with the beneficial effects of physical exercise on diabetic complications.17,18 Our recent study shows that the kidney has its own carnosine metabolism.19 The storage form in the human kidney is anserine (1.1-7.4mmol/kg for anserine). Carnosine and anserine have several protective functions such as buffering of pH, scavenging of reactive oxygen species,20–22 degradation of advanced Glycation end-products,23 inhibition of mesangial cell proliferation,24 and inhibition of TGF-beta‒mediated transcription of extracellular matrix proteins in both podocytes25 and mesangial cells.26 All of these reported properties of carnosine can be beneficial in patients with diabetes. With respect to the genetic predisposition for complications such as diabetic nephropathy (DN), a polymorphism in exon 2 of the CNDP1 gene (homozygosity for 5-5 Leuicine repeats) is associated with low serum concentrations of CNDP1 and a reduced susceptibility for developing DN,27 especially in women with type 2diabetes.28
Under normal physiological conditions the production of ROS (for instance during physical exercise) are neutralised with antioxidants like carnosine and do not result in oxidative damage.29 Any imbalance between these pro-oxidants and antioxidants can result in cellular disruption and damage, and might lead to diseases like diabetes.30 In patients with excessive obesity, pre-diabetes and decreased insulin sensitivity, but without micro vascular complications the storage of carnosine in skeletal muscles has increased.31 In contrast, in patients with type 2 diabetes with micro vascular complications a significant reduction of carnosine in skeletal muscles has been reported, while this reduction remains absent in patients with type 1 diabetes.32 Multiple studies confirmed the hypertensive and antihypertensive effect of carnosine33,34 and the induction of Ca2+ release.35 Considering this vascular effect, we hypothesize that sufficient carnosine storage might result in the prevention of micro vascular complications of diabetes, related to oxidative injury.
Carnosines have been used as food additives for many years in explosion sports such as sprinting, rowing, short track speed skating. Since increased carnosine storage results in reduced muscle pain and fatigue after physical exercise,36–39 many athletes are using beta-alanine as a food supplement. The therapeutic effects of increased carnosine storage have been barely explored in diseases such as diabetes, which are characterized by oxidative injury. Clinical trials are required to investigate the therapeutic potential of these histidine-containing dipeptides in (diabetic) patients. The functional properties of carnosine and anserine and the genetic association with DN indicate that histidine-containing dipeptides have a protective function concerning tissue damage by oxidative stress. Therefore, we hypothesize that higher concentrations of these histidine-containing dipeptides are beneficial in attenuating oxidative injury by quenching oxygen radicals. Experimental studies in rodents already investigated the overall protective effect of carnosine, but species differences need to be taken into consideration because of the absence of CNDP1 in sera of rodents.40 Experiments in diabetic rats and mice have shown that oral administration of carnosine results in prevention of DN25,41,42 and cataract43,44 and they accelerated wound repair.45 Oral supplementation of carnosine in the drinking water of mice with DN results in accumulation of carnosine in muscular tissues and kidneys, reduction of proteinuria, and increased biosynthesis of insulin.25
In humans, taking oral supplements of slow-release beta-alanine tablets drives to de novo biosynthesis of carnosine, leading to increased carnosine storage in tissues.46–50 Carnosines, which are secreted into the circulation by skeletal muscle cells during physical exercise, show similar beneficial effects as physical exercise on diabetic complications.14,51 Enzyme measurements for CNDP1 in human sera of healthy adults and diabetic patients have provided indications for post-translational modifications of CNDP1, which influence its prolonged enzyme activity by additional glycosylation.46 In diabetic patients with microvascular complications, non-invasive measurement of tissue carnosine concentrations by proton magnetic resonance spectroscopy (1H-MRS) shows a significant reduction of carnosine concentrations in skeletal muscles of patients with type 2diabetes, but not in patients with type 1 diabetes.36 Oral administration of β-alanine, the rate limiting amino acid for the biosynthesis of Carnosine,48 to healthy volunteers during 4weeks resulted in reduced fatigue after physical exercise and in 30-50% increase of carnosine storage in peripheral muscle tissue, as determined again by proton-MRS.52 Interestingly, this accumulation is followed by a slow clearance of carnosine during 3-6months, suggesting a prolonged efficacy of short-term intervention.49,53 Next, the latter mentioned study also showed that in humans the histidine-containing dipeptides increase with increasing obesity and glucose intolerance in male individuals. They hypothesize that the skeletal muscle tissue responds differently compared to retina, kidney and liver, because of the high amount of CARNS, which can restore the decreased Carnosine levels more easily in the muscles.31 Still, more research is required to determine the beneficial role of Carnosine in humans.
The results of several experimental studies in rodents as well as human studies indicate the possible protective role of these histidine-containing dipeptides. The ability of carnosine to reserve protein glycation and to inhibit AGEs formation might be the underlying mechanism of the protection in oxidative damage. Still, it is difficult to extrapolate the experimental results into humans because of the lack of CNDP1 in sera of rodents. Microvascular complications of diabetes develop in at least 30% of the diabetes patients. Our hypothesis is that carnosine could play a role in the natural resistance against diabetic damage. We postulate that in the 70% of the diabetic patients no complications occur because of the compensatory increased accumulation of carnosine. In patients with diabetic microvascular complications carnosine storage is not maintained either by insufficient activity of CARNS, or by high activity of CNDP1 in tissue, but more research is needed to confirm these hypotheses. The association between diabetic tissue damage and the improvement after oral supplementation provides evidence for future therapeutic perspectives in treatment of microvascular complications of diabetes.
None.
The author declares no conflict of interest.

©2015 Celine, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.