eISSN: 2471-0016


Research Article Volume 1 Issue 5
1Department of Laboratory Medicine, Umm al Qura University, Saudi Arabia
1Department of Laboratory Medicine, Umm al Qura University, Saudi Arabia
2Department of Clinical Pathology, Mansoura University, Saudi Arabia
2Department of Clinical Pathology, Mansoura University, Saudi Arabia
3Department of Pathology, Mansoura University, Saudi Arabia
3Department of Pathology, Mansoura University, Saudi Arabia
4Department of Clinical Pathology, Suz Canal University, Saudi Arabia
4Department of Clinical Pathology, Suz Canal University, Saudi Arabia
Correspondence: Mohamed El-Boshy E, Department of Laboratory Medicine, Faculty of Applied Medical Sciences, Umm al Qura University, Makkah, Saudi Arabia
Received: January 01, 1971 | Published: December 9, 2015
Citation: Mohamed EE, Husien S, Osama AA, et al. Studies on hepatoprotective effect of pentoxifylline on CCL4 induced liver fibrosis in guinea pigs. Int Clin Pathol J. 2015;1(5):95–100. DOI: 10.15406/icpjl.2015.01.00021
In this study, we investigate the protective and therapeutic effect of Pentoxifylline against liver damage induced by CCl4 in Guinea pigs by monitoring the hepatic m-RNA expression of TGFβ-1. Liver function test and histopathological finding. The CCl4 was orally administrated at dose 1ml/kg bw twice a week for 8 weeks. Pentoxifylline orally administrated at a dose rate at dose 20mg/kg bw/day. We found that Pentoxifylline treatment improved elevated m-RNA expression of transforming growth factor (TGFβ-1), alanine aminotransferase (ALT), total bilirubin (T.Bil), and direct bilirubin induced by CCl4 at 8th week post treatment. Meanwhile, aspartate aminotransferase (AST) and alkaline phosphatase (ALP) are not improved by Pentoxifylline treatment. Finally, we concluded that pentoxifylline has effective in down regulation of chronic liver injury induced by CCl4 if used as protective treatment.
Keywords: aspartate aminotransferase, alkaline phosphatase, M-RNA, Hepatoprotective, glycoproteins, α smooth muscle actin, myofibroblast, peripheral vasodilator, genes, biochemical, reverse transcriptase polymerase chain reaction, spectrophotometer, staining, liver transferase enzyme activities; transcriptional factors, pathology
ALT, alanine aminotransferase; AST, aspartate aminotransferase; T Bil, total bilirubin; ALP, alkaline phosphatase; HSC, hepatic stellate cell SMA, α smooth muscle actin; ECM, extracellular matrix; RT-PCR, reverse transcriptase polymerase chain reaction; BCSR, biotechnology center for service and researches; H&E, hematoxylin and eosin; PTX, pentoxifylline
Liver fibrosis, which ultimately leads to cirrhosis, is a major public health problem resulting from any type of chronic liver injury includes viral hepatitis, alcoholic liver disease, autoimmune hepatitis, metabolic disordered, parasitic disease and nonalcoholic steatohepatitis.1 Hepatic fibrosis is a wound healing response to chronic liver injury which characterized by net accumulation of extracellular matrix (ECM) including collagen, glycoproteins and protoglycan. Hepatic stellate cell (HSC) is the cytological base of the hepatic fibrosis.2 The quiescent HSC are transformed with progressive injury into myofibroblast like cells that characterized by the appearance of cytoskeleton protein α smooth muscle actin (α SMA) which consider a biomarker for HSC activation.2 TGFβ-1 is a key molecule or most fibrogenic cytokines facilitate the activation of HSC and convert it from static HSC into the phenotype of myofibroblast to express α SMA and possess the character of contraction.3 Carbon tetrachloride, CCl4 has been a frequently used chemical to experimentally induced hepatic fibrosis. Depending on the dose and duration the effect of CCl4 on hepatocytes is manifested histologically as hepatic stetosis, fibrosis, hepatocellular death and carcinogenicity.4 The hepatotoxic effect of CCl4 involved in immediate cleavage of CCl4 by cytochrome P450 (CYP2E1) in hepatocytes, which generate trichloromethyle radical leading to lipid peroxidation and membrane damage subsequently resulting in the injury of hepatic parenchymal cells.1
Pentoxifylline, (PTX) a methyl xanthine derivative which is widely used in disorders of vascular perfusion, used as a peripheral vasodilator.5 Recently, PTX is a well known inhibitor of proinflammatory cytokine production, mainly TNF-α.6 Its antifibrotic effect may be due to inhibit TNF-α that stimulated biosynthetic activation of fibroblast, increased collagenase activity, and also through down regulation of profibrogenic cytokines and procollagen I expression.7 This study was aimed to evaluate the hepatoprotective effect of Pentoxifylline on liver fibrosis induced by CCl4 in guinea pigs through detection of gene expression of TGFβ-1, as well as biochemical parameters and histopathological finding.
Experimental animals
Fifty Guinea pigs of 1-2 month old of both sexes were obtained from Helwan farm of Laboratory Animals (Ministry of Public Health). The Guinea pigs were maintained on a pelleted diet and water ad Libitam. The daily requirement of ascorbic acid (50mg / liter of drinking water) was supplied all over the experiment according to Sarah and Maggie.8
Chemicals
CCl4 was purchased from ADWIA Co, Egypt., Primer sequences for PCR amplification:
Transforming growth factor β1 (TGF-β1).
Gene |
Primer sequence |
Base pair |
TGF- β1 |
Sense 5\ TAT AGC AAC AAT TCC TGG CG 3\ Antisense 5\ TGC TGT CAC AGG AGC AGT G 3\ |
162 |
Obtained from metabion international AG., Lend-Christ-Strasse 44\I, Martinsried \Deutschland.
Pentoxifylline (Trental)
Was obtained as coated Tablet contains 400mg pentoxifylline as the active ingredient provided by (Sanofi Aventis. Germany). PTX white to cream crystalline powder, freely soluble in chloroform, methanol and water. It was used as a freshly prepared aqueous solution
Fibrosis induction and treatment
Guinea pigs were divided into 5 groups as follow:Group I (10) served as normal control receive only the vehicle (1ml/kg bw olive oil twice a week for 8 weeks). Group II (10) treated with pentoxifylline (20 mg/kg bw/day all over the experiment) and olive oil. Group III (10) receive 1ml/kg bw of CCl4 diluted 20% in olive oil twice a week for 8 weeks. Group IV (10) pretreated with pentoxifylline for one week before CCl4 administration then treated with CCl4 and pentoxifylline (protective pentoxifylline treated group). Group V (10) after 4 weeks of CCl4 administration and ensure occurrence of fibrosis (5 animals) further treated with CCl4 for 4 weeks and at the same time treated with pentoxifylline (therapeutic pentoxifylline treated group).At the end of 4th and 8th week of CCl4 treatment randomly five Guinea pigs were picked up from each group. Blood samples were collected individually from heart puncture for serum chemistry, Guinea pigs were then sacrificed and specimen from liver was kept in liquid nitrogen for reverse transcriptase polymerase chain reaction (RT-PCR) analyses.
RT-PCR analysis
Total RNA was isolated from Guinea pig livers using the QIAamp extraction method. Preparation of the RNA / primer mix by adding the following (RNA template, Forward Primer and Reverse Primer) to a nuclease-free microtube and mix by pipetting gently up and down. Incubate the mixture at 70-75°C for 5-10 min and place it at room temperature for 5-10 min of denaturation and primer annealing. Prepare the RT-PCR mix and complete it by adding10µl RNA/primer mix, then pipette on ice and mix by pipetting gently up and down. The thermal profile that was used consisted of denaturation at 95°C, annealing at 55-65°C, elongation at 72°C and Final elongation at 72°C. The PCR product was electrophoresed on 2% agarose gel electrophoresis.9 RT-PCR analysis was performed in the Biotechnology Center for Service and Researches (BCSR), Faculty of Vet. Medicine, Cairo University.
Serum biochemical analysis
Prepared frozen serum samples were analyzed for alanine aminotransferase (ALT), asprtate aminotransferase (AST), alkaline phosphatase (ALP), total (T. Bili.) and direct bilirubin (D.Bili.), total protein, albumin (Alb),were determined with a semi-automatic spectrophotometer (BM-Germany 5010) using commercial test kits (Randox Co. UK and Biodiagnostic, Egypt.) according to enclosed pamphlets.
Histopathological studies
Specimens from liver, kidney and spleen were fixed at 10% neutral buffer formalin. Five micron thickness sections were prepared from all specimens and stained by hematoxyline and eosin (H&E). The Hematoxylin and Eosin (H&E) sections were used to select viable representative areas of the fibrosis for subsequent immunohistochemical studies. Also Masson trichrome was also performed on section of the same paraffin blocks in order to confirm and evaluates the degree of fibrosis in Guinea pigs, as well as detection of any concealed minimal fibro-proliferative reaction that could be missed by the H&E examination. All sections were examined microscopically according to Bancroff et al.10
Statistical analysis:
Our results were analyzed by (ANOVA) using the SPSS software statistical program (SPSS for Windows (ver. 15.00, USA). Two groups were significantly different if P value was statistically lower than 0.05.
Biochemical results
Liver transferase enzyme activities, (ALT, AST & ALP) at 4th week post treatment are significantly increased in the CCl4 treated group also in the protective PTX (GP. IV) comparing with Cont. one (Table 1). At 4th week post treatment total bilirubin and direct bilirubin levels are significantly increased in CCl4 treated, protective PTX (GP., III & IV) comparing with the control group (Table 1). Total protein and albumin levels are insignificantly changed between all experiment groups in 4th week post treatment (Table 1). ALT activity at 8th week post treatment is significantly increased in PTX alone, CCl4, protective treated groups (GP.II, IV, respectively) compared with the control group. But in PTX therapeutic group (GP. V) ALT activity insignificantly changed compared with control one, but significantly decreased when compared with CCl4 group (Table 2). AST and ALP activities at 8 week post treatment are significantly increased in CCl4, protective and therapeutic PTX groups (GP., IV, V, respectively) compared with control one (Table 2). Total and direct bilirubin is significantly increased in CCl4 group (GP.III) compared with all examined groups (Table 2). But in protective treated groups (GP. IV), it’s insignificantly change compared either with the control or CCl4 groups.
Histopathological results
Group (I) and (II): Microscopically, the liver of control Guinea pigs and pentaoxifilline treated groups showed normal hepatocytes without degeneration or necrosis, inflammatory cells infiltration and fibrosis besides normal portal areas (Figure 1-2).
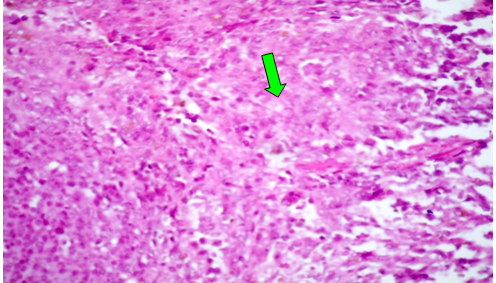
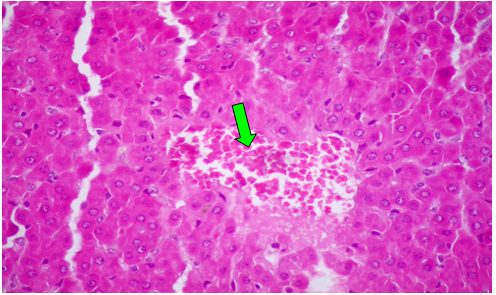
Group (III): AT 4th weeks post treatment with CCl4 alone, the hepatocytes showed severe and diffuse degenerative changes mainly hydropic and ballooning degeneration, (Figure 3). Moreover, focal coagulative necrosis infiltrated with macrophages and lymphocytes were recorded. Moderate fatty changes, represented by sharp, clear vacuoles were seen inside the cytoplasm of some hepatocytes. The portal areas showed congested blood vessels and round cell infiltrations. Focal area of hemorrhages replacing the hepatic parenchyma was also observed (Figure 4). At 8th week post treatment, the observed lesions showed characters of chronicity represented by increased fibrous tissue in the portal areas, around central veins, interlobular and intralobular fibrosis besides hyperplasia of bile duct epithelium (Figure 5). Moreover, some hepatocytes showed nuclear dysplasia. The Masson trichrome stained section revealed blue fibrous tissue in the portal areas, forming thick inter and intralobular septa.
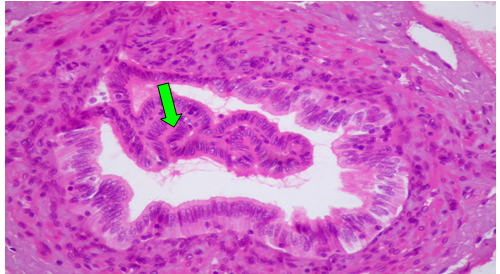
Group (IV): The H&E and Mason trichrome staining section showing attempt of the different organ to return to normal histological structure. At 4th post treatment with CCl4 plus PTX, the hepatocytes showed mild degenerative changes besides necrotic cells (Figure 6). Moreover, congested hepatic blood vessels and sinusoids were noticed. At 8th weeks post treatment the liver showed mild portal fibrous tissue proliferation, biliary hyperplasia and few lymphocyte and macrophages infiltrations (Figure 7-9).
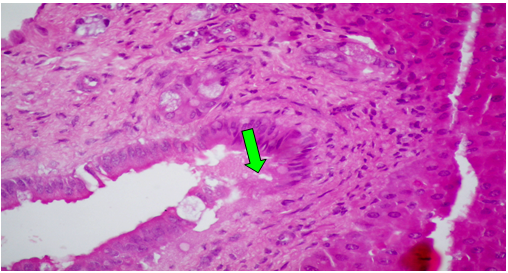
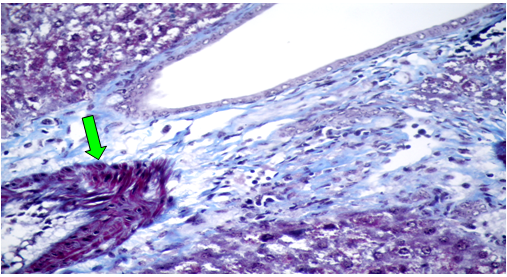
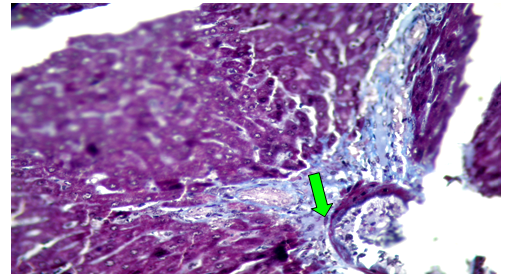
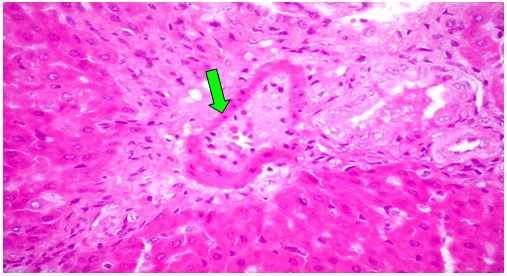
Group (V):At 8 weeks post treatment with PTX and CCl4, the liver showed moderate cloudy and hydropic degeneration, congestion of hepatic sinusoids and blood vessels, few necrotic cells and round cell infiltration in the portal areas (Figure 10). At 4th week PT. At 8th weeks post treatment, the portal tracts showed congestion of blood vessels besides fibrous tissue proliferation around and in portal tracts, infiltrated with few round cells. The Masson trichrome-stained-sections showed mild fibrous tissue proliferation represented by blue coloration of collagen (Figure 11).
CCl4 is an extensively used industrial solvent, and it is the best-characterized animal model of xenobiotic-induced free radical-mediated hepatotoxicity.11 Our study showed that TGF-β1 m RNA expression increased as fibrosis developed in CCl4 induced liver fibrosis in Guinea pigs (82.68% and 88.66 at 4th and 8th week respectively). As TGF-β1 activity is enhanced by proteolytic release and activation of latent TGF-β1 by HSC also other cell such as kupffer cells, invading mononuclear cells, myofibroblast cells and endothelial cells can synthesize and release TGF-β1.12 Pentoxifylline (PTX) has an antifibrotic function and antioxidative property beside its anti-inflammatory effect.13 In our study PTX treatment down regulate expression of TGF-β1 especially protective group at 8th week post treatment. The TGF-β1 m RNA expression was (75.77%) in GP.IV in a 4th week and (36.08% and 47.42%) in (GP.IV and GP.V respectively) at 8th week post treatment with CCl4 and Pentoxifylline. This is agreed with Raetsch et al.5 that suggested that, the antifibrogenic effect of PTX may be due to down-regulated expression of these profibrogenic cytokines. CCl4 administration, causes severe liver damage demonstrated by remarkable elevation of serum AST and ALT levels till the end of the experiment as shown in (Table 1 & Table 2).This elevation may be attributed to the cellular leakage and damaged of structural integrity of the liver cells.11 Also CCl4 treatment induced elevation of serum ALP with high level of total bilirubin, direct and indirect bilirubin (Table 1 & Table 2).Which are considered indicators of cholestasis and pathological alterations of the biliary flow.14
Groups |
ALT |
AST |
ALP |
T. Bili. |
D. Bili. mg/dl |
T.Protein gm/dl |
Alb. gm/dl |
I (Cont) |
20.64c |
29.11c |
10.92b |
0.47b |
0.19b |
4.58b |
3.21a |
II (PTX) |
26.76b |
28.64c |
14.87 a |
0.41b |
0.21b |
5.54a |
3.47 a |
III (CCl4) |
34.19 a |
39.70a |
16.40a |
0.72a |
0.50a |
5.59a |
2.99b |
IV (PPTX) |
30.45 a |
35.30b |
15.50 a |
0.79 a |
0.54 a |
5.63a |
2.89b |
Table 1 Serum biochemical parameters (mean ± S.E) at 4th Week Post Treatment with CCl4 and PTX.
Cont (control), PTX (pentoxifylline), CCl4 (carbon tetrachloride treatment), PPTX (protective PTX), TPTX (therapeutic PTX),
Groups |
ALT |
AST |
ALP |
T. Bili. |
D. Bili mg/dl |
TP gm/dl |
Alb.gm/dl |
I (Cont) |
19.93c |
30.98b |
9.99b |
0.45b |
0.19b |
4.76 |
2.98a |
II (PTX) |
29.89b |
30.49b |
9.27b |
0.52b |
0.21b |
4.51 |
3.37a |
III (CCl4) |
41.76a |
41.07a |
16.93a |
0.80a |
0.50a |
4.9 |
3.92b |
IV |
24.66b |
38.99a |
15.07b |
0.52b |
0.20b |
4.9 |
3.27a |
V (TPTX) |
26.81b |
38.85a |
16.28b |
0.48b |
0.18b |
4.98 |
3.30a |
Table 2 Serum biochemical parameters (mean ± S.E) at 8th Week Post Treatment with CCl4 and PTX.
Cont (control), PTX (pentoxifylline), CCl4 (carbon tetrachloride treatment), PPTX (protective PTX), TPTX (therapeutic PTX),
In our work PTX treated group (GP.II) showed a slight liver damage which detected by elevating levels of serum ALT and ALP at 4th and ALT at 8th week post treatment. Our result agrees with Alexis et al.15 who mentioned that pentoxifylline induced a slight, but not significant, increase of serum ALT (Table 1 & Table 2). Pentoxifylline reduced the degree of hepatocellular injury induced by CCl4 as determined by lower serum levels of ALT at the 8th week PT either in protective (GP.IV) or therapeutic treatment (GP.V), that could be due to anti-inflammatory properties of pentoxifylline (PTX), by decreasing TNF-α production which play an important role in fibrogenesis.6 As well as PTX inhibited nuclear translocation of transcriptional factors, in particular NF-κB which would lead to decreased pro inflammatory mediator synthesis TNFα. In our study The T.Bil., improved by PTX treatment (protective or therapeutic) while Dir.Bili. Return to normal level in the therapeutic treated group at the 8th week PT. In contrast PTX treatment failed to reduce AST, ALP and direct bilirubin, this may explain as, the level of PTX or its metabolites was sub threshold as it only affecting one of the liver function tests and that perhaps a higher dose may alter other liver function test and fibrosis in other animal model.16 This agrees with Raetsch et al.5 who found that PTX did not change the laboratory parameters of cholestasis, suggesting that its antifibrotic properties are unrelated to an effect on hepatobiliary function, but rather due to a direct impact on the effector cells of hepatic fibrogenesis (stellate cells). TP and albumin blood level insignificantly changed by CCl4 treatment except at 8th week post treatment albumin level was increased (Table 1 & Table 2). This is may be due to dehydration as a result of diarrhea caused by CCl4 treatment.17
In our work CCl4 treatment induced several pathological changes including, fatty changes, necrosis and fibrosis. The action of CCl4 upon liver progresses in two phases, firstly ,the toxic effect of CCl4 is due to its conversion by cytochrome P450 to highly reactive toxic free radical which induced lipid peroxidation that resulted in membrane damage , increase permeability to Na+2 and H2O, decrease apolipoprotein synthesis and fatty liver. In the second phase the recruitment and activation of kupffer cell maintain the inflammatory response and liver damage.18 CCl4 known to increase the rate of lipid esterification, synthesis of fatty acid, triglycerides and cholesterol from acetate resulting in steatosis.19 Finally, we concluded that pentoxifylline is to somewhat effective in down regulation of chronic liver injury induced by CCl4 if used as protective treatment. Further research recommended for more definitive knowledge about the efficacy of pentoxifylline as a hepatoprotective agent.
None.
The author declares no conflict of interest.

©2015 Mohamed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.