eISSN: 2471-0016


Case Report Volume 3 Issue 1
1Department of Pathology and Human Anatomy, Loma Linda University Medical Center, USA
1Department of Pathology and Human Anatomy, Loma Linda University Medical Center, USA
2Department of Pathology and Laboratory Medicine, University of California at Davis Health System, USA
2Department of Pathology and Laboratory Medicine, University of California at Davis Health System, USA
Correspondence: Justin Kerstetter, 11234 Department of Pathology and Human Anatomy, Loma Linda University Medical Center, Loma Linda, Tel 909-558-4094, Fax 909-558-4189
Received: January 01, 1971 | Published: October 27, 2016
Citation: Lei L, Raghavan R, Chen M, et al. Primary cuetaneous hodgkin lymphoma: a revisit and case report. Int Clin Pathol J. 2016;3(1):177–181. DOI: 10.15406/icpjl.2016.03.00067
Background: Primary cutaneous Hodgkin lymphoma is a rare entity of debate. Methods and Results: We report a case of iatrogenic primary cutaneous Hodgkin lymphoma that initially regressed after methotrexate withdrawal but recurred thirteen months later. While reviewing seven idiopathic and two additional iatrogenic cases of primary cutaneous Hodgkin lymphoma in the literature, some common features were noted, including older patients and chest wall sparing. Idiopathic primary cutaneous Hodgkin lymphoma follows a relatively indolent clinical course but has the potential to progress to systemic disease. Iatrogenic primary cutaneous Hodgkin lymphoma is highly associated with Epstein-Barr virus infection. The two iatrogenic cases reported in the literature were managed with immunosuppressant withdrawal, chemotherapy plus surgery or radiation, and both patients were disease free on follow-up for ten months and six years respectively.
Conclusion: The recurrence of disease in our case suggests that discontinuation of immunosuppressant alone is not enough for the management of iatrogenic primary coetaneous Hodgkin lymphoma. Considering the complications of standard chemotherapy and radiation, a less intensive intervention may be appropriate and warrants further evaluation.
Keywords: cutaneous hodgkin lymphoma, epstein-barr virus, iatrogenic, methotrexate, recurrence
Classical Hodgkin lymphoma (HL) typically involves lymph nodes, particularly cervical and mediastinal lymph nodes. Skin involvement is seen in 0.5% to 3.4% of cases.1–3 The first reported cutaneous HL was probably a case secondary to systemic disease described by Thomas Hodgkin himself in 1832, as stated by Fernandez-Flores A.4 It is usually seen in stage IV disease and is associated with dismal prognosis, though it may follow an indolent course if systemic disease is well controlled.5–7 Secondary cutaneous HL was not uncommon before the advent of modern chemotherapy. However, primary cutaneous Hodgkin lymphoma (PCHL) is an entity of much debate and worthy of further discussion. We herein report a case and present a comprehensive review of the literature.
A 58-year-old Hispanic woman presented with a 3-month history of a painless, non-healing skin ulcer on the right forearm. She also had some other ulcerating but self-healing cystic skin lesions. Past medical history was significant for a 6-year history of dermatomyositis that had been treated with methotrexate (MTX) 10 to 15 mg a week, Cell Cept (mycophenolate mofetil) 1000 to 1500 mg a day, and prednisone 5 mg a day. The cumulative dose of MTX was estimated to be roughly in the range of 3120 to 4680 mg. Physical examination showed a 4.0 x 3.0 cm ulcerated area located on the dorsal surface of the right arm just proximal to the elbow.
An excision was performed. The excised specimen was a 4.0 x 2.5 cm ovoid piece of tan skin cut to a depth of 1.0 cm. A central 1.5 x 1.0 cm ulcerated lesion was present, at least 0.5 cm from all surgical margins. The specimen was fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 3- to 4-µm and stained with hematoxylin-eosin (H&E) or counterstained with hematoxylin for immunohistochemical studies (IHC). IHC and Epstein-Barr virus-encoded small RNAs (EBER) in situ hybridization were performed with adequate positive and negative controls.
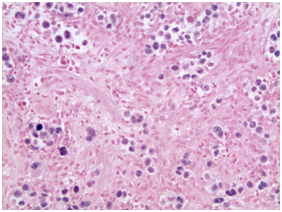
The H&E stained sections showed a dense dermal infiltrate without epidermotropism. The epidermis was ulcerated but otherwise unremarkable. The dermal lesion showed a diffuse, infiltrative growth pattern with some necrosis (Figurers 1a & 1b). Tumor foci are not surrounded by rim of small lymphocytes (Figurers 1b & 1c). At high magnification, it consisted of scattered large atypical cells in a background of mixed inflammatory cells including small lymphocytes, plasma cells, neutrophils and rare eosinophils. The atypical cells demonstrated large nuclei with vesicular chromatin, prominent inclusion-like nucleoli and ample amphophilic cytoplasm. Some of them had bilobed mirror-image nuclei. The large atypical cells were morphologically consistent with Hodgkin/Reed-Sternberg (HRS) cells and their variants (Figure 1c & 1d). Frequent mitotic Figureures, including atypical forms, are also noted (Figure 1d).
The large atypical cells were strongly positive for CD15 (Figure 2a) and CD30 (Figure 2b), dim positive for PAX5 (Figure 2c), but negative for CD45 (Figure 2d), BOB.1, Oct-2, CD19, CD20, CD3, CD5, CD43 and Bcl-2. CD3 and CD5 highlighted background small T cells. IHC for Ki-67 showed a high proliferation index of almost 100% in the large atypical cells. Melanoma and carcinoma were ruled out based on negative staining for S-100, melanoma cocktail, cytokeratin cocktail and CAM5.2. EBER in situ hybridization was positive in the large nuclei of HRS cells and their variants (Figure 3). Small lymphocytes were EBER negative. The morphology and immune-profile were most consistent with a classical Hodgkin lymphoma. A full clinical workup including a complete physical exam, chest, abdomen and pelvic CT scans, and a bone marrow biopsy was negative for involvement elsewhere except the skin lesion. A diagnosis of iatrogenic immunodeficiency associated primary cutaneous Hodgkin lymphoma was made. The surgical margins were involved.
The ulcer healed well after withdrawal of MTX. The patient was then placed on active surveillance with no chemotherapy or radiation. Thirteen months after MTX withdrawal, the lesion recurred locally. A biopsy was performed. The specimen was a 0.7 x 0.7 cm piece of tan skin cut to a depth of 0.2 cm, with a 0.3 x 0.3 cm friable papule abutting the surgical margins. Pathology examination showed the same morphology and immunohistochemical profile, and EBER was positive. The patient was, unfortunately, and then lost to follow-up.
Both the initial excision specimen and the biopsy of the recurrent lesion in our patient showed morphologic and immunonphenotypic features characteristic of classical HL. Lack of systemic disease on follow up excluded secondary cutaneous involvement and confirmed that the cutaneous lesion was the primary. Though PCHL is a controversial entity, the recurrence of the disease after MTX withdrawal supports the diagnosis of PCHL in the present case.
Mechanisms of secondary cutaneous involvement by HL include direct extension from underlying nodal disease, retrograde lymphatic spread, and rarely hematogenous metastasis. As a result, the chest wall is the most common site of involvement.8 Other cutaneous manifestations of systemic HL include erythroderma, eczematoid or psoriasiform eruptions, pruritus, urticaria, prurigo-like papules, acquired ichthyosis, xeroderma, Addisonian hyperpigmentation, alopecia, recurrent herpes zoster infection or vasculitis. These nonspecific cutaneous issues are much more common with a prevalence rate of 3% to 53%. They represent a paraneoplastic syndrome, but skin biopsy may be needed to exclude cutaneous HL.9
In contrast, PCHL is exceptionally rare. By definition, PCHL is cutaneous HL with no evidence of extracutaneous disease at the time of diagnosis. Even in cases presenting with cutaneous HL, workup often revealed lymphadenopathy.6,10–12 Of note, HL relapse can present as cutaneous lesions in patients with minimal systemic disease.13,14 On the other hand, PCHL may have lymph node metastasis as evidenced by sinus infiltration other than effaced nodal architecture, and a positive lymph node does not necessarily exclude PCHL.15
Since Kren and Doesekker first described PCHL in 1919, as reported by Fernandez-Flores A,4 there has long been a debate about whether PCHL truly exists until Sioutos reported five cases confirmed with immunohistochemical studies in 1994.15 Due to considerable morphologic and immunophenotypic overlap with a variety of other entities such as anaplastic large cell lymphoma and lymphomatoid papulosis, many cases initially thought to be HL had diagnoses revised upon further review.16–18 It is particularly problematic when the lesion is limited to atypical sites like skin.19 An expanded immunohistochemical panel is sometimes needed to confirm the diagnosis.16,20 For instance, dim PAX5 positivity and weak to no expression of Oct-2 and BOB.1 favor classical HL. All the three markers are almost invariably absent in T-cell lymphomas, and are strongly expressed in B-cell lymphomas.20 In immunocompromised patients, mucocutaneous ulcer, a more indolent entity, must be considered.21 Features supporting mucocutaneous ulcer include a broad band of T-cells concentrated at the base of the lesion, frequent plasmacytoid apoptotic cells, and wide range in nuclear size of the Epstein-Barr virus (EBV) positive cells.22,23 These features are absent in our case. In short, a diagnosis of PCHL cannot be made without thorough clinical investigation and careful histologic as well as immunohistochemical evaluation.
A more strict though somewhat arbitrary definition of primary cutaneous lymphoma has been proposed as cutaneous lymphoma with no evidence of extracutaneous disease within the first 6 months after diagnosis.24 Given the rarity of skin as a primary site of HL as well as the relatively indolent clinical course of cutaneous lymphoma, this definition is probably more appropriate for PCHL. An example is patient number 4 in Sioutos’s case series.15 The patient was found to have nodal HL two months after cutaneous presentation. Of note, in contrast to most other PCHL cases with lesions located on the extremities or scalp, this is the only case with lesion on the chest wall, a typical location for secondary cutaneous HL. Following this strict definition, a PubMed search retrieved nine cases of PCHL, including seven idiopathic cases and two iatrogenic cases (Table 1).15,25–29
|
Age/Sex |
Race |
Past medical history |
Location |
Treatment |
Outcome |
EBV |
Ref. |
Idiopathic |
||||||||
1 |
54/M |
White |
No |
Left lower leg |
Radiation |
Nodal MCHD 6y later |
N/A |
15 |
2 |
52/M |
White |
No |
Right forearm |
No |
Recurrent skin lesion, 20y |
N/A |
15 |
3 |
17/M |
Black |
No |
Right thigh |
Topical steroids |
Recurrent skin lesion, 9y |
N/A |
15 |
4 |
45/F |
White |
No |
Forearms, legs |
Chemo |
Progressing/recurrent skin lesion for 3y; Disease free for 5y post chemo |
N/A |
15 |
5 |
78/F |
N/A |
No |
Bilateral arms |
No |
Nodal MCHD 2y later |
Pos |
22 |
6 |
86/M |
Black |
No |
Left ankle |
Radiation |
Nodal LPHD and NHL 1y later |
N/A |
23 |
7 |
70/M |
N/A |
No |
Right back |
Radiation |
Disease free, 7y |
Neg |
24 |
Iatrogenic |
||||||||
1 |
74/M |
N/A |
UC, on infliximab |
Scalp |
W, excision, chemo |
Disease free, 10m |
Pos |
25 |
2 |
25/F |
N/A |
DM, on MTX/ thalidomide/steroids |
Scalp |
W, chemo, radiation |
Complete remission, 6y |
Pos |
26 |
3 |
58/F |
Hispanic |
DM, on MTX/ CellCept/steroids |
Elbow |
W, excision |
Recurred 13m later |
Pos |
Present case |
Table 1 Clinicopathological features of primary cutaneous hodgkin's lymphoma
Chemo, chemotherapy; DM, dermatomyositis; EBV, epstein-barr virus; F, female; LPHD, lymphocyte predominant Hodgkin disease; M, male; MCHD, mixed cellularity Hodgkin disease; MTX, methotrexate; N/A, not available; Neg, negative; NHL, non-Hodgkin’s lymphoma; Pos, positive; Ref, references; UC, ulcerative colitis; W, withdrawal of immunosuppressive medications
Clinically, though PCHL can occur in young adults, it does not demonstrate a typical bimodal age distribution as its nodal counterpart. Most of the patients are in their fifties to seventies. Interestingly, the same finding also applies to primary intracranial HL.30 Sites of involvement are usually confined to the extremities followed by scalp, in contrast to chest wall in secondary cutaneous HL. The lesions may be solitary or multiple. Manifestations vary from painless erythematous papules, nodules, and plaques to ulcers.
The clinical course of idiopathic PCHL is variable but relatively indolent. Four of seven patients (57%) had either been disease free or had recurrent skin lesions during follow-up for up to 20years. However, three of seven patients (43%) developed nodal HL within 1 to 6years. If the relaxed definition of PCHL is applied, nodal HL can develop as soon as 2months after the cutaneous manifestation.15 Patients may also be at increased risk for other lymphoproliferative disorders including non-Hodgkin lymphoma.26 Predictors of disease progression remain unknown at this point. Personalized treatment and close follow-up are warranted.
Among various iatrogenic/MTX-associated lymphoproliferative disorders, diffuse large B cell lymphoma is the most common, followed by HL.31,32 To the best of our knowledge, only 64 cases of iatrogenic immunodeficiency-associated HL have been reported.30–33 Skin is the third most common site after lymph nodes and intestine. In contrast to the EBV positivity of approximately 50% in sporadic classical HL, 76% of iatrogenic HL cases are EBV positive.29 In keeping with the finding, all three iatrogenic PCHL cases were EBV positive. The strong association can be explained by the fact that, in the setting of iatrogenic immunodeficiency, lack of immune surveillance allows the proliferation of EBV-transformed B lymphocytes.
In terms of prognosis, idiopathic PCHL has a nearly 50% chance (three out of seven patients) of progressing to systemic disease. Although it is impossible to draw a conclusion on the prognosis of iatrogenic PCHL based on three cases available, no progression to nodal HL has been reported, suggesting a more indolent clinical course compared to idiopathic PCHL. This is probably because the triggers are clear and can be removed in iatrogenic PCHL.
Regarding the management, no consensus is available. Idiopathic PCHL needs at least some treatment including surgery and/or chemoradiation as well as close follow up given the potential of disease progression. As for iatrogenic PCHL, the treatment followed what had been practiced for iatrogenic lymphoproliferative disorders. It is well established that a subset of these cases can undergo spontaneous regression after cessation of immunosuppressive therapy, particularly the ones that are EBV positive.32,34,35 Ichikawa et al.32 reported that, though 59% of iatrogenic lymphoproliferative cases regressed after MTX withdrawal alone, 46% of them had recurrence and/or residual disease later and chemotherapy and radiation were needed.32 Cessation of immunosuppressant alone is apparently not enough to address the issue of recurrence. Additional chemotherapy and/or radiation appear necessary. This also holds true for iatrogenic PCHL, as the lesion in our patient regressed after MTX withdrawal but recurred 13months later. The patient reported by Vieites underwent six cycles of lomustine-etoposide-prednisone after surgery and had no recurrence for at least ten months.28 Another patient reported by Loo did not respond to MTX withdrawal, and therefore received 4 cycles of ABVD and local radiation. She was in complete remission during 6 years of follow-up.29 The main concern is whether standard chemotherapy and radiation are justified or an overtreatment for PCHL. Other issues that need be considered when giving these patients standard chemo-radiation include, a) patients can die of complications of chemoradiation, and b) they may die of unrelated disease given the old age and relatively indolent clinical course of their lymphoma.32,36 The intensity of chemoradiation still remains a question.
In conclusion, we report a case of PCHL and summarize common features of this entity based on all of the ten cases that have been reported to date. Given the potential of disease progression in idiopathic PCHL, the potential of recurrence in iatrogenic PCHL as well as complications of standard chemo-radiation, a personalized, less intensive treatment may be appropriate for patients with PCHL. Further investigation is warranted on a larger series of cases to obtain a better understanding of this unique entity.
None.
The author declares no conflict of interest.

©2016 Lei, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.