eISSN: 2471-0016


Research Article Volume 4 Issue 6
1Department of Pathology, University of Rochester, USA
1Department of Pathology, University of Rochester, USA
2Department of Pathology, First Medical Lab, Jordan
2Department of Pathology, First Medical Lab, Jordan
Correspondence: Hussam Abu Farsakh, Department of Pathology, University of Rochester, Rochester, New York and First Medical Lab Director, 5th circle, near Sheraton Hotel, Building no. 14, Amman, Jordan
Received: January 01, 1971 | Published: June 21, 2017
Citation: Farsakh HA, Ijmail A. Morphological and histochemical changes in muscle biopsies of myasthenia gravis with a typical clinical presentation. Int Clin Pathol J. 2017;4(6):170–173. DOI: 10.15406/icpjl.2017.04.00116
The morphological and histochemical changes in muscle biopsies from 6cases with myasthenia gravis (MG) were studied, The patients were misdiagnosed at the initial presentation, however they were all proved later by either histochmical and serological tests or improvement on mestinone therapy to have MG. Here, the histomorphological and histochemical changes that were seen in muscle biopsies of MG with atypical clinical presentation were the followings: atrophy of type II fibers; predominance of type I fibers; increased NADH staining at the neuromuscular junction (NMJ). The most consistent finding is marked reduction in acetylcholinesterease (AChE) activity at the end plates in all cases. The results of this study suggest that histochemical and acetyl cholinesterase stains, in combination with clinical assessment can help to identify unsuspected cases of MG.
Keywords: muscle biopsies, myasthenia gravis, acetyl cholinesterase receptor
NMJ, neuromuscular junction; MB, muscle biopsies; AChE, acetylcholinesterease; MG, myasthenia gravis; NADH-TR, tetrazolium reductase; SDH, succinic dehydrogenase; AChE, acetyl cholinesterase; AChRA, acetylcholine receptor antibody
Myasthenia gravis (MG) is a relatively rare autoimmune disorder in which antibodies form against acetylcholine nicotinic postsynaptic receptors at the neuromuscular junction of skeletal muscles.1,2 The disease is characterized by variable weakness and fatigability of oculobulbar and limb muscles.3 It has a bimodal peak of incidence with first peak in the third decade and the second peak in the sixth decade.4 The diagnosis is usually made on clinical and electrophysiological grounds. In the majority of patients, initial presentation is due to the involvement of extra ocular muscles. With the progression of the disease, facial, bulbar, proximal limb muscles, neck extensors, and diaphragm get involved.5–7
This disease has a prevalence of 2/10000 population. Untreated MG has a 10year mortality of 20-30% and 85% of the patients are seropositive for acetyl choline antibodies.8,9 The histomophology and histochemical description of MG in muscle biopsy is rare. This may be due to the fact that most of the patients are diagnosed by clinical examination, serology, EMG and nerve conduction studies. However, in some cases, the clinical picture may not be typical and serology tests may either be negative or not available. In this article, we are describing the muscle morphological and histochemical changes of MG. Knowing these changes are essential for neuropathogiest so as not to miss cases with atypical presentation. We would like also to emphasize the value of routine use of acetylcholinesterease (AChE) staining in muscle biopsy.
We studied 6 patients who visited The First medical Lab in Amman, Jordan, during a 5 year period. Age ranged from 11 to 60 years (5F, 1M).
Clinical evaluation
None of patients had an initial diagnosis of myasthenia gravis at the time of presentation. Data were collected regarding muscle power, pattern of weakness, functional abilities, EMG studies, acetylcholine receptor antibodies.
Muscle biopsy
The muscle biopsy was performed using 14 gauge tru cut needle through a small knife opening of 2 mm in diameter in the skin overlying the right quadriceps muscle, under local anesthesia. More than 30 passes were performed on each patient to collect enough muscle tissue material. The material was wrapped in sponge dampened with saline. A block with frozen O.C.T compound is prepared on a metal chip. All muscle material were mounted on the metal chip and covered by the O.C.T compound. A metal beaker filled with isopentane was dipped in liquid nitrogen for container. The mounted biopsy is then immersed in the cooled isopentane for 15 seconds. The frozen biopsy was removed and rapidly placed in -20 C freezers. Later, the muscle was being cut by the cryostat by 5µm. The cut tissue was stored at -20 freezers for final staining.
Morphological investigations
Diagnostic needle muscle specimens were obtained from the quadriceps or the deltoid muscles of all patients. Standard histological and histochemical analysis was performed for the specimens of all patients as described.10 5µm serial cross-sections were cut in a cryostat. Sections were stained with hematoxylin-eosin, modified trichrome stain, NADH tetrazolium reeducate (NADH-TR), succinic dehydrogenises (SDH), myofibrillar ATPase at pH 10.6., 4.2, 4.6, and acetyl cholinesterase (AChE).
Clinical examination
Table 1 show that we have two peaks of occurrences. One peak at the second decade: five patients (age 11-21 years), and another peak at the sixth decade one patient (age 60).
Patient |
1(mn23.4c) |
2 (mn18.4b) |
3 (mn11.4c) |
4 (mn17.4c) |
5(mn21.4c) |
6(mn2.5b) |
Age(yrs)/sex |
12/F |
15/F |
11/M |
60/F |
21/F |
20/F |
Duration of symptoms before diagnosis (yrs) |
11 |
2 |
10 |
6 |
5 |
1 |
Pattern of weakness |
proximal muscles |
proximal |
Proximal muscles |
extra ocular muscles |
neck muscles |
proximal muscles |
Ptosis |
absent |
absent |
Present |
present |
present |
absent |
Diurnal variation |
none |
none |
none |
none |
none |
none |
CPK |
normal |
normal |
Normal |
normal |
normal |
mildly raised |
Family history |
no |
no |
Yes (one brother) |
no |
no |
no |
Consanguinity |
no |
no |
Yes |
no |
no |
no |
EMG |
myopathic |
myopathic |
myopathic |
myopathic |
myopathic |
myopathic |
AChR Antibody |
negative |
negative |
Negative |
negative |
negative |
negative |
Response to therapy |
Mestinon improved |
Mestinon followed by thymectomy improved |
Mestinon improved |
Mestinon improved |
Mestinon improved |
Mestinon improved |
Table 1 Clinical details and laboratory features of the six patients
Histological Analysis
Routine histological analysis of the quadriceps or deltoid muscle specimen of all cases showed mild variation of fiber size with interspersed mildly atrophic fibers (Figure 1). The abnormal variability of fiber size with type I fibers predominance was clearly demonstrated in Figure 2 and were seen in cases no. 1,4 &5. The histochemical examination of the muscle biopsies of all patients revealed marked reduction in ACHE activity (Figure 3). There was increased oxidative enzyme NADH reaction at the neuromuscular junction in all cases but case no. 3 (Figure 4). Table 2 shows the characteristics of the histochemical changes in six myasthenia gravis patients. The histological pictures in all cases showed almost common features of the following: minimal muscle fiber variation in size even in cases with long standing myasthenia gravis, and showed occasional atrophied fibers. In three of the six patients, predominance of type I fibers were observed, the atrophied fibers tended to be of type II. The most consistent finding in all cases was marked reduction in AChE staining at the end plates, However, increased NADH at the end plate were seen in five of the six patients. None of the cases showed lymphorrhagia that had been described before. In three of the six patients, electron microscopy was performed. In two of three cases mild increase in glycogen was noted and in one case, glycogen increase was coupled with increased fat globules.
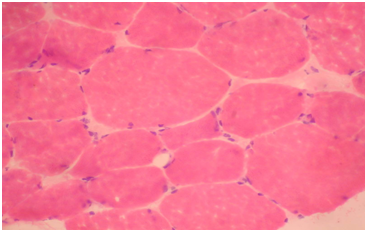
Figure 1 Quadriceps muscle biopsy of patient 1. Hematoxyline and eosin sections showing variation in fiber size with interspersed mildly atrophic fibers. The atrophic fibers are seen selectively in type II fibers (Mag.400x).
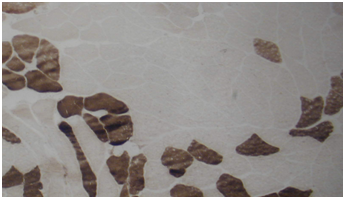
Figure 2 Quadriceps muscle biopsy of case 1 with alkaline preincubation ATPase stain at pH 10.6 showing predominance of type 1 fibers (Mag.400).
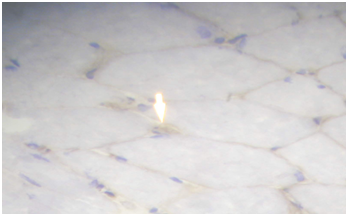
Figure 3 Quadriceps muscle biopsy of case 2 showing marked reduction of AChase activity (Mag. 400x).
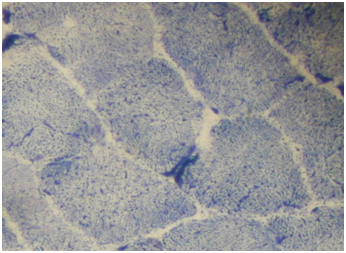
Figure 4 Quadriceps muscle biopsy of case 2 showing muscle fibers with increased NADH staining at the NMJ.
Acetylcholine receptor antibody (AChRA) was positive only in one case out of six cases (16%). All patients responded dramatically to Acetylcholinesterease inhibitors (like mestinon) and thymectomy was performed in case no.2. Thymic hyperplasia was seen on microscopic examination.
Patient |
1(mn23.4c) |
2 (mn18.4b) |
3 (mn11.4c) |
4 (mn17.4c) |
5(mn21.4c) |
6(mn2.5b) |
Muscle biopsies |
R. Quadriceps |
L. deltoid |
R. Quadriceps |
R. Deltoid |
R. Quadriceps |
R. Quadriceps |
Atrophic fibers |
type II |
type II |
type II |
both types |
type II |
type II |
NADH stain at Neuromuscular junction |
increase |
increase |
normal |
increase |
increase |
increase |
Fiber type predominance |
type I |
type I and II |
type I and II |
type 1 |
type I |
type I and II |
Muscle necrosis |
no |
no |
no |
no |
no |
no |
Muscle inflammation |
no |
no |
no |
no |
no |
no |
Lymphorrahgia |
no |
no |
no |
no |
no |
no |
Centronucleation |
<5% |
<5% |
10% |
<5% |
<5% |
<5% |
Fibrosis |
no increase |
no increase |
no increase |
no increase |
no increase |
no increase |
Acetyl cholinesterase at neuromuscular junction |
decrease |
decrease |
decrease |
decrease |
decrease |
decrease |
Table 2 Summary of the morphological changes in MG patients
The six cases of myasthenia gravis reported in this study showed some features similar to those reported in the description by Dubowitz.9–11 Except for Dubowitz description, the recent literature is lacking significantly, any other descriptive features of histochemical staining for MG. In our study, the six patients constitute 3.3% of 182patients observed in a 5-year period. All patients represented a diagnostic challenge. For them, different diagnoses other than MG have been previously proposed, including myositis, motor neuron diseases, limb girdle muscular dystrophies, or mitochondrial myopathies. Understanding that MG can have atypical clinical presentation is very important for their diagnosis. While 90% of patient with MG are reported to have AChRA12 patients with atypical clinical presentation that come to muscle biopsy may have much less chance of having positive AChRA. In our series, only one out of six cases (16%) had positive AChRA In that case, his clinician ordered the AChRA only after we made the diagnosis based on histochemical and AChE staining. The inability of recognizing the morphological changes of MG by the pathologist, render diagnosing such cases correctly almost impossible. In our series, the duration of patients’ symptoms ranged from 1 year to 11years. We strongly emphasize the use of AChE staining in any case that the pathologist sees minimal variation of muscle fibers disproportionate to the muscle power of the patient. The decreased AChE staining has been reported in Washington’s University web site,13 and we agree with their report.
Thymic hyperplasia is noted in 75% of patients with MG. Of these, germinal hyperplasia is noted in 85% and thymic tumor in 15%.3 Thymectomy, a known modality for the treatment of MG, induced excellent response in case no.2. None of the other cases had thymectomy. None of our patients had typical diurnal variation seen in MG with better power in the morning. Most of the affected muscles were the facial muscle, neck muscle and proximal upper and lower limb muscles. This selective pattern of muscle involvement may hypothesize a selective functional alteration of neuromuscular transmission.14 The pathological mechanism leading to selective proximal muscle weakness and wasting in MG patients may be related to the presence of unknown auto antibodies or specific immunologic profile.14,15
It is interesting to report that lymphorrhagia which has been described by Russell, Adams et al.,16 Engel & McFarlin,17 is not seen in any of our 6cases and it is not a constant finding.16,17 Dubowitz has emphasized the presence of type II atrophy with patchy distribution in 4 out of 6cases of his study, he noticed type I fiber atrophy in some areas in 4 out of 6 cases. He suggested that the reason for these patchy changes were not all motor units myasthenia.9–11 The increased NADH staining at the NMJ has been described by Rodolico as increased tubular aggregates at the NMJ in patient with MG.14 We observed this finding in 5 out of 6 sixcases, and helped us in making the diagnosis in these cases. However, this finding is not specific and has been observed in cases other than MG in our entire cases. In conclusion, MG is a common muscle disease, but it comes to muscle biopsy only rarely because most of these patients are diagnosed based on clinical and serology grounds only. Pathologists may miss the diagnosis of MG if he is not aware of the changes that occur in such cases or AChE staining is not available in his lab.
None.
The author declares no conflict of interest.

©2017 Farsakh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.