eISSN: 2471-0016


Case Report Volume 2 Issue 3
1Department of Neurology, Georgetown University Medical Center, Washington DC, USA
1Department of Neurology, Georgetown University Medical Center, Washington DC, USA
2Children?s of Alabama, University of Alabama at Birmingham, USA
2Childrens of Alabama, University of Alabama at Birmingham, USA
3Departments of Pathology and Neurology, Georgetown University Medical Center, USA
3Departments of Pathology and Neurology, Georgetown University Medical Center, USA
Correspondence: Brent T Harris, Department of Neurology, Georgetown University Medical Center, Building D, Room 207, 4000 Reservoir Road, NW, Washington DC, 20057, Tel 202-687-5345
Received: January 01, 1971 | Published: May 24, 2016
Citation: Khan GA, Sadeghi S, Whittaker B, et al. Congenital amyelinating neuropathy: a neuropathology case report and review of prior cases. Int Clin Pathol J. 2016;2(3):52–56. DOI: 10.15406/icpjl.2016.02.00040
Congenital hypomyelination/amyelination diseases of the peripheral nervous system can appear in the neonate or in late infancy with pathological findings of either hypomyelination or no myelination. Patients with these disorders most often present with severe hypotonia, weakness, delayed motor development, and frequently succumb to respiratory failure. The first reported case of congenital hypomyelination was described in 1969. Most cases have mutations in one of several genes encoding for proteins involved in peripheral nerve myelination (MPZ, PMP22, MTMR2 and Sox 10). Both familial and sporadic cases are reported. We describe here a case of an 18 year old female who had a long history of failure to thrive and developmental delays. She met all milestones for her development until about 5 years of age, when she began having difficulty maintaining normal weight. Her cardinal symptoms were chronic pain, autonomic instability, nutritional intolerance, developmental delay, and foot drop. She had no family history of similar neurological disease. Her neuropathological findings from sural nerve and skeletal muscle biopsies show amyelination, mild loss of large axons, and uniform atrophy of myofibers. This case represents one of the oldest living individuals at time of biopsy for amyelinating disease described in the literature.
Congenital hypomyelinating neuropathy (CHN) is a rare congenital neuropathy characterized by non-progressive weakness, areflexia, hypotonia, slow nerve conduction velocities, and hypo-, or in some cases, amyelination. Patients usually present in infancy and may die within the first several years of life, usually after infectious complication. Infantile CHN presents later and patients survive several years.1 Biopsy in amyelinating neuropathies usually shows a diminution of axon numbers. The histological hallmark is striking absence of myelin around nearly all axons, some perhaps showing a few myelin lamellae. 2The first reported case was published in 1969 by Lyon.3 Subsequently, several cases have been reported that further describe the clinical and histopathological/ultrastructural features (Table 1). In most of the cases, patients were diagnosed within one year of life and died at an early age. Our patient was not known to have CHN until, at age 18, she had a muscle and sural nerve biopsy which provided the diagnosis.
Case |
Phenotype |
Biopsy Findings |
References |
Case: 1 |
Born in healthy parents. She was hypotonic. She stood with support at the age of 18 months and at 30 months of age; she was not able to walk by herself. |
Almost total disappearance of myelin sheaths, apparent preservation of axons and an increase in collagenous material. |
|
Case: 2 |
A 3 year and 11 months - old Japanese male with hypotonia, delay motor development and muscle weakness. |
Absence of myelin sheath. Most of the large myelinated fibers demonstrated “onion bulb”. |
|
Case: 3 |
A male child aged 3 years and 10 months with muscle weakness and wasting associated with delayed motor development. |
Muscle biopsy showed denervation findings and sural nerve biopsy showed normal size axons with no or very thin myelin sheath enclosed by empty basement membrane. |
|
Case: 4 |
A male child aged 3 years and 11 months with delayed motor development and weakness. |
Muscle biopsy shows denervation findings and sural nerve biopsy shows near total absence of myelinated axons. |
|
Case: 5 |
Hypotonia at 10 months of age. |
Sural nerve biopsy revealed abnormally thin myelin sheaths with mild reduction in the number of myelinated fibers. |
|
Case: 6 |
Hypotonia and developmental delay identified at 5 months. At 5 years of age patient developed lower extremity weakness and had ataxic gait. |
Sural nerve biopsy revealed severe hypomyelination with abnormal onion bulb. |
|
Case: 7 |
At 12 months of age, delayed motor mile stones, hypotonia and scoliosis then developed muscle wasting and sensory ataxia. |
Loss of myelin fibers, thin myelin sheaths, atypical onion bulbs. |
|
Case: 8 |
Newborn with severe hypotonia with decreased respiratory effort. |
Sural nerve biopsy showed nearly complete loss of large diameter myelinated fibers and scattered axon with very thin myelin sheaths. |
|
Case: 9 |
Patient presented with hypotonia, arthrogryposis of the left hand and neuromuscular respiratory failure at birth. |
Sural nerve biopsy exhibited large axon with little to no compact myelin and few basal lamina onion bulb. |
|
Case: 10 |
Floppy infant and delayed motor mile stone. At age 12 years, unable to walk. |
Lack of normally myelinated fiber. Basal lamina onion bulbs. |
|
Case: 11 & 12 |
New born baby girl hypotonia, arthrogryposis, difficulty in swallowing. Her father was 27 years old with history of hypotonia. |
Father: absence of myelin sheaths on light microscopy. In EM showed no myelinated fibers, with the exception of a single fiber with a very thin layer of myelin. Daughter: Sural nerve biopsy not performed. |
|
Case: 13 |
At 5 months of age, hypotonia and failure to thrive. |
Sural nerve biopsy showed nearly complete absence of myelination of both large and small fibers. |
|
Case: 14 |
At 3 years old, hypotonia and gross motor delay. |
Sural nerve biopsy showed a severe decrease in the number of large myelinated fibers. |
|
Case: 15 |
Hypotonia at age 4 months, gastrostomy at age 11 months, ventilation at age 4 years. |
Severe reduction of myelinated fibers, reduplicated basal lamina around thin myelinated fiber. |
|
Case:16 |
18 years old female with a long history of failure to thrive and developmental delay. |
Sural nerve and muscle biopsies showed complete loss of myelin around axons and marked atrophy of myocytes. |
Current case |
Table 1 Prior case reports of Congenital Hypomyelination Neuropathy.
The patient at time of biopsy was an 18 year old white female with a longstanding history of failure to thrive and developmental delay. She met all milestones until about 5 years of age, when she began having difficulty maintaining normal weight. At age 13 a wrist xray for bone age suggested a delayed bone development age. She was treated by an endocrinologist for possible growth hormone deficiency due to small stature and low weight, though the growth hormone therapy did not prove successful to maintain her growth. Laboratory studies, focused primary on nutritional and endocrine functions, were largely normal, though plasma metanephrines were sometimes found to be 1.5-2 times normal values. Creatinine phosphokinase studies were never obtained and muscle and nerve conduction studies never performed. She subsequently developed severe scoliosis, requiring hardware placement that was removed later due to infection. Initial presentation of the patient to Children’s of Alabama (COA) occurred as abdominal pain with vomiting that “turned black” without diarrhea, and she complained for several days of fever, back pain, foul odor of urine. Radiology evaluation revealed pneumotosis and markedly distended stomach into the pelvis. She was treated with TPN followed by trans-pyloric then oral feeds. She achieved minimal weight gain. She was ruled out from superior mesenteric artery syndrome and carnitine deficiency. At one point, mitochondrial neurogastrointestinal encephalopathy (MNGIE) was considered, but this was much later in her course, and genetic testing did not confirm the diagnosis. She was discharged and transferred to an adolescent treatment facility in Virginia for treatment of complex medical problem and a suspected eating disorder. At age 18, a decision was made to place a G-tube, and at that time an observant surgeon suggested a nerve and muscle biopsy should be performed. The biopsy was sent to Bostwick/American International Pathology Labs and consultation requested from Neuropathology at Medstar Georgetown University Hospital. A diagnosis of congenital amyelination/hypomyelination with associated myopathy was given. Genetic studies to look for specific myelination related gene defects were not undertaken. Shortly after biopsy, she returned to Children’s of Alabama with respiratory failure requiring ventilator support. She struggled with intermittent ventilator associated infections, worsening pulmonary status, dysrhythmias, chronic malnutrition and deconditioning. An attempt to wean her from the ventilator resulted in immediate desaturations and bradycardia leading to a code event with chest compressions and pharmacological intervention. After consulting with a neurologist, the family decided to withdraw care and allowed her to pass peacefully. Permission to perform an autopsy was not obtained.
Pathological findings
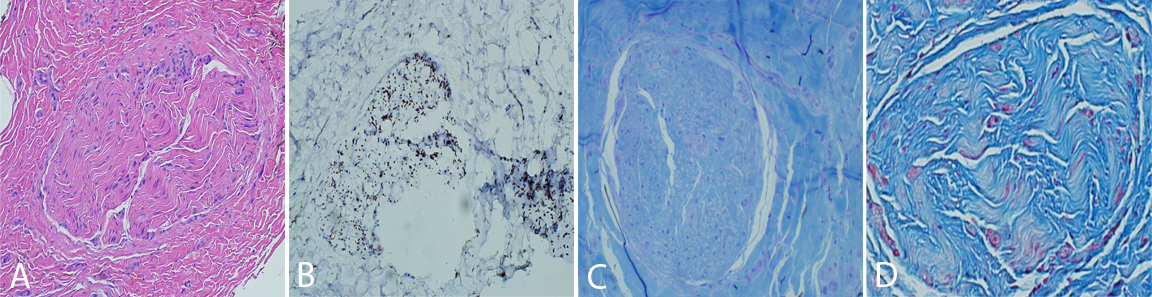
The nerve and muscle specimens from the lower extremity were received and processed per standard muscle and nerve workup protocols including enzyme histochemistry, special stains for nerve and muscle, nerve teasing, and electron microscopy. H&E stained sections of the nerve biopsy show cross sections of sural nerve and surrounding connective tissues (Figure 1A). Blood vessels show no evidence of vasculitis, and Congo red stain displays no evidence of amyloidosis. There is significant fibrosis of the peri-, epi -or endoneurium observed. These are best highlighted with trichrome stain (Figure 1D). Trichrome also shows no large myelinated fibers. A neurofilament immunostain displays evenly distributed and moderately diminished complement of axons (Figure 1B). One micron thick, toluidine blue stained sections display cross sections of several nerve fascicles. This stain shows a complete absence of thickly myelinated fibers (Figure 1C). No supernumerary Schwann cell accumulations are seen. Endoneurial vessels have enlarged endothelial cells. Ultrastructural analysis of these sections confirms the observations seen on thick sections (Figure 2). There is no discernible thick or thin myelin present around axons. Unmyelinated fibers have loosely arranged membranes around them, and Schwann cell nuclei are present. No supernumerary Schwann cells are present (Figure 2). The tease preparation displays strands of fibrous tissue without any discernable myelinated fibers.

Hematoxylin and eosin stained frozen sections of skeletal muscle show cross sectional arrays (Figure 3A). The connective tissues and vessels are unremarkable. The majority of fibers are uniformly atrophied ranging from 8-25 µm in cross sectional diameter. The trichrome stain fails to highlight any ragged red fibers and does not show an increase in endomysial connective tissue. Intramuscular nerves are not seen on trichrome. Acid and alkaline phosphatase staining patterns are normal. NADH shows no abnormalities. PAS stain fails to show any increase in glycogen deposition. The oil red O shows normal lipid deposition. ATPase staining displays type II fiber predominance but no clear fiber type grouping of both fiber types. Congo red staining fails to highlight any amyloid deposition. COX/succinate dehydrogenase staining shows occasional COX negative fibers. Esterase staining is mildly increased for all fibers (Figure 3B). Myophosphorylase staining is normal.
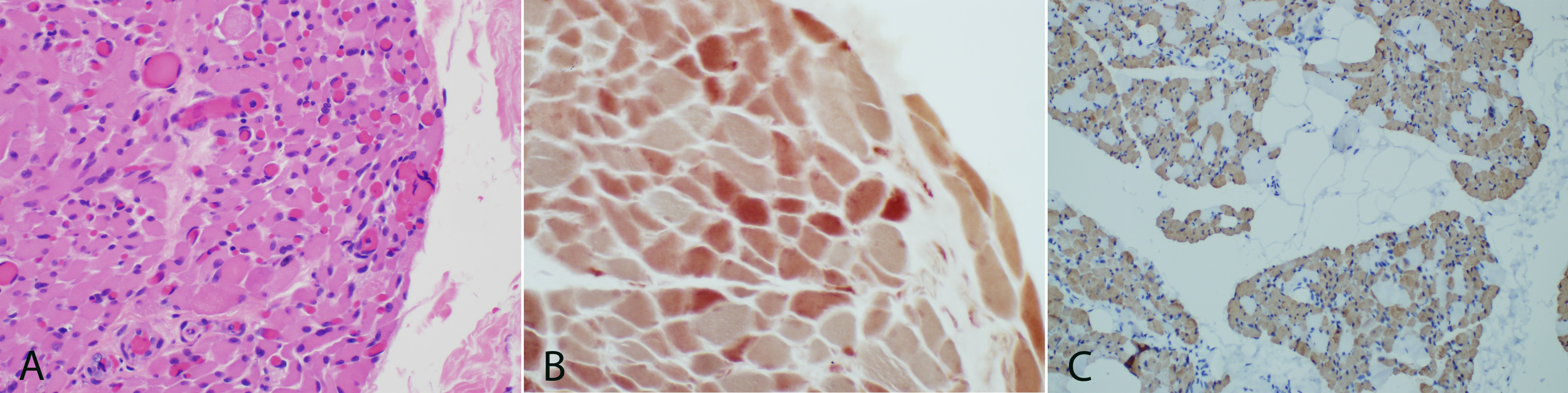
One micro thick toluidine blue stained slides show atrophied muscle and connective tissues. Ultrastructural studies display muscle with normal glycogen and lipid content, normal structure and location of mitochondria, and no inclusions. The myofibrillar architecture is markedly disrupted and the normal myofilament Z-banding pattern is abnormal in many areas (Figure 4). Cytoplasmic bodies are also observed. H&E paraffin embedded sections show skeletal muscle in cross sectional and longitudinal array. Normal appearing perimysial connective tissues are seen. The myofibers are markedly and relatively uniformly atrophied with some variability in size and shape. Most show rounded contours, but some are acutely angulated. Immunohistochemical stains for fast myosin and slow myosin highlight a type 2 predominance of muscle fibers but a fairly normal checkerboard pattern of type-2 and type-1 fibers (Figure 3C). Immunostaining for major histocompatibility complex (MHC 1) fails to highlight any significant increase in staining. The finding of complete loss of myelin within the sural nerve and marked atrophy of myofibers confirms the diagnosis of congenital amyelinating/hypomyelinating neuropathy and myopathy.
Congenital hypomyelination neuropathy, is a hereditary or sporadic demyelinating neuropathy first reported in 19693 describing a baby girl born to healthy parents. Clinically, she was hypotonic and was able to stand with support at 18 months of age. Her sural nerve biopsy revealed almost total disappearance of myelin sheaths, apparent preservation of the axons, and an increase in collagen deposition. In 1985, Harati and Butler described microscopic features of congenital hypomyelination neuropathy based on several cases with histological and ultrastructural features of small intramuscular nerves and sural nerves.4 Most of the reported cases of CHN describe markedly decreased myelin, no active myelin breakdown, preservation of axons, no well-formed Schwannian onion bulbs, and absence of other demyelination - remyelination processes. A case reported by Ono in 1982, describes a muscle biopsy showing irregularity of muscle fiber size and endomysial fibrosis. Absence of myelin sheaths of intramuscular nerves was found in the trichrome stain. Myosin ATPase reaction stains revealed a deficiency of type 1 fibers and predominantly type 2A and 2B muscle fibers.5 In 1986, Seitz reported a hypomyelination neuropathy in a female newborn where histology of muscles showed marked variation of the muscle fiber diameters with a range between 3 and 15micrometers,6 which is similar with our case’s muscle findings.
CHN patients can present in two different ways. One group presents in the neonatal period with severe hypotonia, weakness, and frequently develops respiratory failure. The other group presents after the neonatal period, usually with hypotonia and delayed motor development.1 For all types of cases, the diagnosis is established by clinical history, signs and symptoms, EMG, and sural nerve biopsy. All reported cases with biopsies describe complete loss of myelin sheath or markedly diminished myelination with intact axons. CHN and Dejerine-Sottas syndromes have similar clinical symptoms but have distinct differences on sural nerve biopsy findings.7 Dejerine-Sottas syndrome patients’ nerve biopsies show hypomyelination with demyelination/remyelination changes with continuous myelin destruction, and classic onion-bulb formation. Nerve biopsies of patients with CHN show hypomyelination of most or all fibers but lack of inflammation and demyelination/remyelination changes.4 CHN is believed to affect only peripheral myelination, though no autopsies with central nervous system examination have been reported in the literature. Several mutated genes (MPZ, PMP22, EGR2, MTMR2 and SOX10, and MPZ) encoding peripheral nerve myelin proteins have been identified in congenital hypomyelinating neuropathy, Dejerine-Sottas syndrome and Charcot Marie Tooth including late onset axonal CMT2.8 Among those genes, myelin basic protein zero (MPZ) is the most frequent cause of CHD. Myelin basic protein zero is a major component of peripheral nerve myelin. It is a trans membrane glycol protein which is essential in the compaction of myelin.1 Shapiro and his group describe the normal myelination process with the myelin sheath wrapping around the axon and the extracellular component of P0 on opposing myelin membrane forming tetramers that adhere to each other.9 A mutation within a critical portion of P0 might disrupt this interaction. The location and type of mutation within P0 may determine the clinical phenotype.10
In 2008, Smit and his group published a case report about a family affected by congenital hypomyelinating neuropathy.11 They described a daughter and father with similar apgar-scores and hypotonia with other associate symptoms when they were born. They found in a sural nerve biopsy from the 27year old father no myelin sheath on light microscopy.12–16 Electron microscopy showed no myelinated fibers with the exception of a single fiber with a very thin layer of myelin. Both father and daughter were tested for mutations in demyelinating neuropathy associated genes and were both found to be heterozygous for an insertion of a G nucleotide in exon 4 of the MPZ gene.
Our patient was an 18year old female with a longstanding history of failure to thrive and developmental delay for unknown reason during most of her life. She met all milestones until about 5years of age, when she began having difficulty maintaining normal weight. Her neuropathological findings on sural nerve and muscle biopsies shortly before her death showed complete loss of myelin around axons and marked atrophy of myocytes. This is consistent with the previously reported cases and confirms the patient had a congenital amyelinating/hypomyelinating neuropathy. To the best of our knowledge she was one of only two adults to receive this diagnosis after biopsy in the medical literature.
None.
The author declares no conflict of interest.

©2016 Khan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.