eISSN: 2469-2778


Case Report Volume 12 Issue 1
1Oncological Surgery Service, General Surgery Service, Mexico
2Spanish Hospital of Mexico City, Mexico
Correspondence: Gallardo Navarro Elias, Oncological Surgery Service, General Surgery Service, Mexico, Tel 2227642945
Received: December 20, 2023 | Published: January 11, 2024
Citation: Elias GN, Mario GRF, Carlos MS, et al. Myxoid liposarcoma of the right lower limb. Hematol Transfus Int. 2023;12(1):9-11. DOI: 10.15406/htij.2023.12.00321
It is known that the diagnosis of these tumors can be delayed due to the lack of specific symptoms, and this delay in diagnosis and the large size that these lesions can reach significantly influence the prognosis and treatment of the disease, sometimes putting in danger, the functional preservation of the affected limb. The main objective in the treatment is to preserve the limb. We present a case of myxoid liposarcoma located in the right thigh in a 57-year-old patient who presented to pain in the right lower extremity.
Keywords: liposarcoma, soft tissue tumor, radiotherapy
Both benign and malignant soft tissue tumors present as a soft tissue mass that does not allow differentiation between the two, until the moment of histopathological diagnosis. In general, patients attend with the physician between four and six months after the appearance of the first symptom, which in general includes pain and tumor.1 Surgical experience has shown that local recurrence varies according to the type of surgical procedure: 90% after resection only, 39% after wide resection, 25% after soft tissue resection and 7-18% after amputation. Overall recurrence after radical surgery has varied from 30 to 20% in the last 30 years, with an overall survival of 17%.2 The fine needle biopsy is performed with a 21-23 gauge needle, aspirating cells from the tumor mass and a smear is made for cytological evaluation. This has the disadvantage that, being a cytological study, the pathologist will be unable in most of them to establish the grade and histological type.3 To make the diagnosis we must use different imaging methods. Simple radiography is of little use, it can demonstrate an increase in soft tissues, usually non-specific. Ultrasounds, due to their great availability and safety, are increasingly used and are usually capable of confirming the presence of a specific soft tissue mass, allowing the anatomical location and solid, liquid or mixed nature of the tumor to be assessed. In addition, it is useful for directing sample collection in case of fine needle aspiration or tru-cut biopsy. Both computed tomography (CT) and magnetic resonance imaging (MRI) are useful and are recommended for the pre operative evaluation of lesions and the follow up after treatment; there are no great advantages in either technique. MRI allows sagittal and coronal planes to be made and better differentiates muscles and vessels, often more reliable, demonstrating the invasion of adjacent planes, also not giving radiation to the patient. Positron emission tomography (PET) is also being used for this pathology, but its true applications have not yet been defined; A recent meta-analysis on PET published by Bastiaannet concludes that its routine use cannot be justified.4 6In general, tumors located in depth, larger than 3 cm, solid or heterogeneous should not be excised without a prior biopsy, either by puncture or incisional. Soft tissue tumors must be evaluated by a team of specialists in musculoskeletal pathology, who will be the ones to carry out the definitive treatment of the patient. This team should consist of oncologists, oncology orthopedists, diagnostic imaging specialists, and pathologists with specific training in musculoskeletal tumors. The steps to follow must be planned jointly in order not to jeopardize subsequent treatment, which, if possible, will be conservative with limb salvage. Teamwork allows everything that happened to be planned preoperatively in such a way that it does not harm the final treatment, thus reducing the percentage of recurrences and local complications. Finally, the surgical specimen with which a definitive diagnosis will be made must be studied with its histological grade (given mainly by histological parameters such as the degree of cellular differentiation, mitotic index and areas of necrosis) and the surgical margins. These elements, together with clinical data, will allow staging the patient and establishing a prognosis.3,5,6
A 57-year-old female who begins with a tumor in the right pelvic limb (thigh) of 2 and a half years of evolution of approximately 2 x 2 cm accompanied by lacerating pain (muscle tear) 4/10 in the Numeric Pain Rating Scale (NRS) of gradual onset in the posterior region of right thigh, without irradiation. Subsequently, she reported a progressive increase in volume increasing up to 10 x 7 cm in length (Figure 1). Currently, the pain increased to 8/10 and worsens when sitting. Currently she cannot tolerate sitting, a simple CT scan of the lower limbs where requested (Figure 2). Given the time elapsed and the lack of improvement, Computed Tomography Angiotomography (CTA) was performed in Figure 3 where the existence of an increase in density and a subfascial tumor adjacent to the right femoral biceps measuring 7.4 x 7.5 x 4.3 cm was observed. The patient was approached by compartmentectomy of the affected area with free margins and subsequently underwent radiotherapy in the tumor area. The pathological diagnosis was myxoid liposarcoma (Figure 4 & 5).
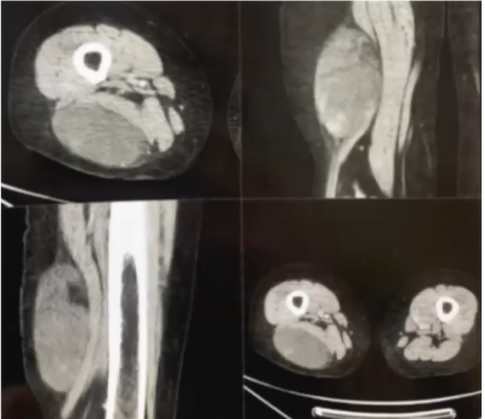
Figure 2 Subfascial tumor adjacent to the right biceps femoris measuring 7.4 x 7.5 x 4.3 cm without alterations of the arterial system.
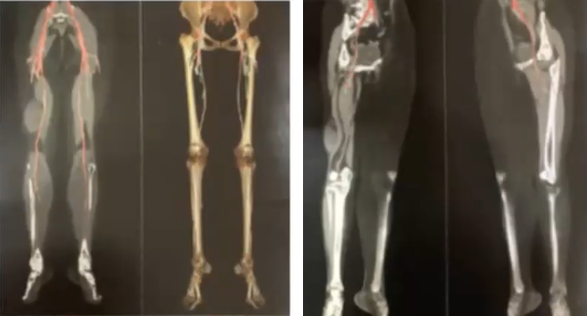
Figure 3 Angiotomography: Subfascial tumor adjacent to the right biceps femoris measuring 11.4 x 7.9 x 4.3 cm with a volume of 201 CC.
Soft tissue sarcomas are rare and arise from mesenchymal tissues. They can occur anywhere in the body, and represent 1% of all cancers in adult.7,8 60% originate in the extremities, but it is three times more common in the lower limbs than in the upper limbs, 30% are located in the trunk and 40% are retroperitoneal.9 The latter occur in 10% of cases due to hematogenous metastasis and 90% de novo.8 Liposarcoma arises from adipocyte precursors, representing 10–20% of all soft tissue sarcomas, most commonly found in the extremities and retroperitoneum.10 Clinically, soft tissue sarcomas represent a heterogeneous group that have in common the appearance of a soft tissue mass that increases in size, measures more than 5 cm and becomes painful. The more of the characteristics mentioned the lesion has, the greater the risk of malignancy.11 The tumor can limit the range of motion or cause numbness or pain if pressure is placed on nearby anatomical structures, as mention in this case.10 In the liposarcoma group we find 4 different types: well-differentiated, dedifferentiated, pleomorphic and myxoid liposarcoma. Despite having little vascularization, the percentage of metastasis, its prognosis and difficulty for surgical removal varies depending on the histological type. Regarding prognostic factors, it is important to mention the relationship with the aponeurotic planes and the size of the primary tumor, as well as cellular differentiation, the number of mitoses and the extent of necrosis.10 Well-differentiated liposarcoma or atypical lipomatous tumor have a low degree of malignancy that rarely produces metastasis, but strict monitoring of the patient must be carried out because it can cause recurrence,7 in the same way a poorly differentiated liposarcoma can appear again or in 10% of cases can come as a complication of a pre-existing well-differentiated tumor and can be low or high grade.12 Poorly differentiated liposarcoma is defined as a lesion lacking adipogenic differentiation with the degree of differentiation based on cellularity and atypia. 65% of cases occur in the retroperitoneum and 20% in extremities.12 It is a very aggressive tumor with a local recurrence rate of 41%, a metastasis rate of 17%, a mortality rate of 28%7 and a 5-year survival rate of approximately 55%, which is defined by the absence of disease progression and not by the degree of response to treatment.11 Myxoid liposarcoma is usually an indolent disease; a small percentage of patients develop soft tissue metastases years after the initial diagnosis, which represents a worse prognosis. It must be managed aggressively to improve survival since local recurrence will occur whenever they are not well encapsulated or completely resected.9 In the previously presented clinical case, a diagnosis of pleomorphic liposarcoma was made, which is the subtype with the greatest percentage of malignancy, presenting local recurrence of 37% and metastasis in 10 years of 41%, a percentage that increases directly with the size of the lesion. Furthermore, it has a 10-year survival of 39%, compared to the other subtypes that have a survival of 87% when they are well differentiated and 76% in myxoid liposarcomas.9
The prognosis is closely related to the extension to adjacent structures and we recommend en bloc surgical resection with free margins confirmed by intraoperative study, so myxoid liposarcoma falls within the group of sarcomas with adipose differentiation, since within myxoid liposarcomas We find tumors with different behavior and clinical evolution.
None.
The authors declare that there is no conflicts of interest.
None.

©2024 Elias, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 World Thalassemia day is observed on May 8th. Thalassemia is a genetic blood disorder that causes the body's hemoglobin level to be lower than normal, which makes you feel exhausted. This year the main motto of this day is to Empower Lives, Embracing Progress: Equitable and Accessible thalassemia treatment for all. Related submissions received for this event will be offered with 30% discount towards publication.
World Thalassemia day is observed on May 8th. Thalassemia is a genetic blood disorder that causes the body's hemoglobin level to be lower than normal, which makes you feel exhausted. This year the main motto of this day is to Empower Lives, Embracing Progress: Equitable and Accessible thalassemia treatment for all. Related submissions received for this event will be offered with 30% discount towards publication.