eISSN: 2469-2778


Research Article Volume 6 Issue 2
1Nova Scotia Health Authority, Canada
2Dalhousie University, Canada
3McGill University, Canada
4Canadian Blood Services, Canada
5King Abdullah Medical City, Saudi Arabi
Correspondence: Hanadi Aljedani, Queen Elizabeth II Health Sciences Center, Halifax Infirmary hospital, 1796 Summer Street. Halifax, Nova Scotia, B3H 3A7, 6th Floor, Blood transfusion Services, Canada, Tel +1 (902)440-5688,
Received: February 08, 2018 | Published: March 23, 2018
Citation: Aljedani H, Sadek I, Liwski R, et al. Effective enforcement of plasma transfusion policy. Hematol Transfus Int J. 2018;6(2):60-64. DOI: 10.15406/htij.2018.06.00154
Background and objectives: Despite clinical policies and guidelines established to ensure appropriate use of plasma, published studies revealed that a considerable number of plasma transfusions are still inappropriate. At our institution, an evidence-based clinical policy for plasma transfusion was implemented October 2012 and then reinforced by May 2014. The policy indicates the clinical situations when plasma transfusion is considered appropriate and when it is considered inappropriate and suggests alternative therapeutic modalities. Clinical situations in which plasma units will be issued as requested without being triaged include therapeutic apheresis procedures, actively bleeding patients, massive transfusion protocol, and intra-operative patients.
Materials and methods: Retrospectively, from October 1st, 2011 through November 30th, 2016, data on all transfused plasma units were collected and analyzed. Plasma units that were issued without being triaged were excluded from the data pool.
Results: Total of 3545 plasma units was transfused during the whole study period. Plasma transfusions were reduced by 39.5% after implementing the enforcement policy by May 2014, which saved 234,045 Canadian dollars. There was a 40.1% reduction in hours of work for blood transfusion services staff. No significant changes regarding mortality rates in hospital in-patients and patients in emergency departments, excluding intra-operative and trauma patients, were seen. The same was true in terms of the average length of in-patient hospitalization before and after the enforcement of the policy. After the policy enforcement, plasma-related transfusion reactions were reduced by 47.5 %.
Conclusion: The enforcement of a policy to guide plasma transfusion practice in a hospital have been found to be effective, cost-efficient, and pragmatic. It is recommended that health care centers have measures in place to audit transfusion practice periodically.
Keywords: fresh frozen plasma, transfusions medicine, quality management, transfusion reactions, enforcement of policy
INR, international normalized ratio; LIS, laboratory information system; AP, apheresis plasma; FP, frozen plasma; PCC, prothrombin complex concentrates; FC, fibrinogen concentrates
Plasma transfusions are used to replace deficient/dysfunctional coagulation factor(s) when the patient is significantly bleeding or whenever significant bleeding is anticipated and a specific coagulation factor concentrate is not available for use or in emergency situations when precise laboratory diagnosis is not possible. Most of coagulation factors need to be at a level of 25-30% to maintain adequate coagulation system.1 Plasma transfusion at a dose of 10-15 mL per kg body weight is expected to increase coagulation factors by 10-20%.2‒4 After plasma transfusion, coagulation factor levels increase temporary and then decline to reach the baseline level within 6 to 24 hours.5 The effect of plasma transfusion on international normalized ratio (INR) is transient; for the same volume of transfused plasma, a greater reduction in INR is observed when INR is more than 1.8.6,7 Despite implemented policies and guidelines, published studies showed that plasma transfusion may be unjustified in up to 34% of the studied populations.8‒10
The available studies showed that strict implementation of appropriate guidelines or policies for plasma transfusion is considered effective. A retrospective study by Traves et al.11 revealed that strict application of guideline and education of physicians reduce plasma transfusion by 80%.11 A Prospective study by Sarode et al.12 revealed that implementation of triage for plasma transfusions orders resulted in 60% reduction of plasma transfusions.12 The concerns about unjustifiable plasma transfusions is because of the risk of transfusion-associated adverse reactions, including transfusion-related acute lung injury (TRALI), transfusion-associated circulatory overload (TACO), severe allergic reactions and other complications that may add morbidity and mortality risk to the recipients. In addition, there are limited resources for plasma units to keep with the increasing demand and costs.13
Plasma units collected by the Canadian Blood Services are either derived from single whole blood donation (FP) that is separated from the red blood cells and buffy coat into 200-300 ml bags or derived from apheresis donations (AP) that are collected into 500 ml bags. Other sources of plasma include cryosupernatant plasma (CP) that is prepared from slowly thawed FP and Solvent/Detergent plasma which is manufactured outside Canada and provided to hospitals for special indications.14
Nova Scotia Health Authority developed a multidisciplinary clinical manual policy for plasma transfusion guidelines was approved and implemented on October 2012 and was updated and enforced by May 2014. The enforcement policy clearly indicates when plasma transfusion is considered appropriate and when is not. All plasma transfusion requests will be triaged at blood transfusion services with exception of few clinical situations. A color-coded flowchart was created for Blood Transfusion Services staff to follow when plasma transfusion request received. When plasma transfusion is deemed not justified as per guideline, a consultation with hematopathologist may be indicated. Plasma transfusion requests will be processed as requested without being triaged in certain urgent clinical situations, including plasma transfusion requests for therapeutic apheresis, bleeding trauma patient in emergency department, massive transfusion protocols, and plasma requests for intra-operative patients.
Data on all transfused plasma units were collected for the period of October 1st, 2011 through November 30th 2016, from the hospital Laboratory Information System (LIS). The data transferred into Excel and de-identified. The collected data included type of transfused plasma products, indications for plasma transfusion and the location where transfusion occurred. Data on Prothrombin Complex Concentrates (PCC) and Fibrinogen concentrates (FC) transfused in the same study periods were collected from the hospital LIS.
The data were analyzed and compared for the two study periods: 30 months before and 30 months after the date of enforcement of plasma transfusion policy, May 2014.
Exclusion criteria include all plasma units transfused for patients with clinical situations where plasma transfusion requests will not be triaged.
The cost of plasma products was calculated in Canadian Dollars based on Canadian Blood Services prices per plasma product types for the corresponding year of production. Data about total number of in-patients and emergency room admissions and mortality rates in our center for the study duration were obtained from health information department. Transfusion reactions associated with plasma transfusions over the study periods were collected from LIS and transfusion reaction records at hospital transfusion services. Transfusion reactions associated with plasma transfused for patients in the exclusion criteria were not included in the study.
Chi-Square analysis was used to assess the significance of difference of results before and after enforcing the plasma transfusion policy. P value less than 0.05 is considered significant.
The primary objective of the study is to evaluate if the initiation of enforcement policy at May 2014 was effective in reducing the number of inappropriate plasma transfusions. The secondary objectives were to evaluate effect of enforcement of the policy on the work load, cost and transfusion reactions.
Total number of plasma transfusions in the whole study periods, including plasma transfusions for the exclusion criteria, was 16,188 units. The total number of transfused plasma units included in the study was 3545 units, representing 22% of all transfused plasma units in our institution for the study period. The plasma units transfused for patients in the exclusion criteria were 78% of total plasma transfused in our institution for the last 5 years.
The two plasma products types were used in the studied population are AP and FP. The other types of plasma products; Solvent/Detergent plasma and CP, are strictly used for therapeutic apheresis procedures which are excluded from our analysis. The total number of transfused AP units was 3085 (87%), whereas total number of transfused FP units was 460 (13%), Table 1. The total number of plasma transfusions reduced from 2208 in the first study period to 1337 in the second study period, equivalent to a 39.5% reduction (P value < 0.0012).
Type of plasma products |
Total number of plasma units (%) |
Number of units |
Decrease in number of units |
|
1 Oct 2011– 30 April 2014 |
1 May 2014– 30 Nov 2016 |
|||
AP |
3085 (87) |
1886 |
1199 |
36.40% |
FP |
460 (13) |
322 |
138 |
57.10% |
Total |
3545 (100) |
2208 |
1337 |
39.40% |
Table 1 Comparison of types and number of transfused plasma products over the study periods
The monthly average of transfused plasma units (AP and FP combined) dropped from 74 units per month in the first study period to 41 units per month in the second study period, Figure 1.
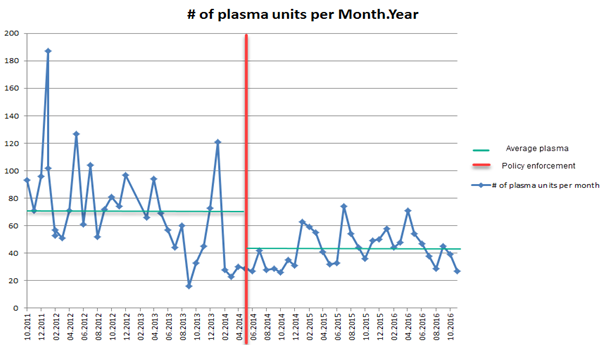
Figure 1 illustrates the monthly average of transfused plasma units (AP and FP combined) in blue lines pre- and post-enforcement of the policy at May 2014, red line. The average number of monthly transfused plasma (green line) dropped from 74 units per month in the first study period to 41 units per month in the second study period. The figure demonstrates the variations in the total number of transfused plasma units per month in the 1st study period compared to the 2nd study period which could be due to variability in the practice of plasma transfusions before enforcing plasma transfusion policy.
For the first study period, a total of 891 patients received plasma transfusions with average of 2.4 units per patient. Even though the majority of patients in the first period (92%) received a total of 4 units or less, the number of units transfused in the first period ranges from 1 to 81 units per patient with a standard deviation of 4.6. In the second study period, 554 patients received plasma transfusions with average of 2.4 units per patient and about 90% received 4 units or less. However, the range of plasma units transfused was 1 to 30 units per patient with a standard deviation of 2.8. These variations are probably due to variability in the practice of plasma transfusions and lack of standardization before enforcing plasma transfusion policy. Comparing the two study periods, the data revealed that fewer patients got almost the same amount of plasma. Thus, the reduction in the amount of transfused plasma in the second period is expected to be as a result of on change in the indication for plasma transfusions rather than plasma dosage.
To note, some patients may need multiple plasma transfusions over the study periods for chronic disease as in chronic liver disease or congenital factor deficiencies which may explains the wide range of number of units for plasma transfusions per patient.
The patient’s weight is not available in our current LIS version and therefore we were not able to retrospectively evaluate if the plasma doses requested for our patients were acceptable as per guidelines. The policy indicates that plasma transfusion in a dose of 10-15 ml/kg is considered appropriate.
Following the Canadian institute for health information 2015, the time needed for selection, inspection, thawing, assigning, labeling and issue one unit of plasma is 7.1 minutes. By enforcing the policy, the total hours of work were reduced by 40.1%, Table 2. Even though the study showed that enforcing the policy resulted in reduction in hours of work, there were no available records for documentation of time required for communication between blood transfusion services staff and clinical team or hematopathologists which may jeopardize accurate calculations of reduction of hours of work for blood transfusion staff. In addition, it was not possible to analyze the effect of reduction in plasma transfusion on workload for clinical staff, nurses, or patients. It is presumable that reduction in plasma transfusion would have saved time for clinical staff and nurses in regard time for administration of plasma units, checking vital signs for the patients and processing the required documentations.
The reduction in plasma transfusions saved about 234,045 Canadian dollars with a decrease of 33.7% (P value = 0.99), compared to the costs of transfused plasma units in the first period, Table 2.
Study period |
Total cost |
Work load time |
Transfusion reactions |
1 Oct 2011‒ 30 Apr 2014 |
6,93,802.00 |
261.28 hours |
61 |
1 May 2014‒30 Nov 2016 |
4,59,757.00 |
158.21 hours |
32 |
Total reduction (%) |
234,045.0 (33.7%) |
103.07 (40.1%) |
29 (47.5%) |
Table 2 Comparison of the cost of transfused plasma units, workload required to process the transfused plasma and the incidence of transfusion reactions between the two study periods
There was a decrease in the number of reported transfusion reactions associated with plasma transfusions by 47.5%, Table 2. There were no reported cases of definite or possible TRALI in both study periods however, the number of reported cases of definite or probable TACO related to plasma transfusion did not change significantly for both study periods, 1 case in each study period. The incidence of allergic reactions associated with plasma transfusions were decreased in the second period compared to the first study period (24 vs 46) presumably because less units of plasma have been transfused in the second period.
The use of Prothrombin complex concentrates (PCC) did not change significantly between the two study periods. However, the use of Fibrinogen concentrates (FC) increased by almost 6-folds in the second study period, 181 vials in 1st study period vs 1094 vials in the second study period. Initially, in the first study period, FC products were used strictly for certain indications including patients with congenital hypofibrinogenemia. Cryoprecipitate products were used for cases with hypofibrinogenemia due to acquired causes like massive transfusion, liver diseases, and during or after cardiac surgery procedure. Cryoprecipitate products need to be ABO group compatible or matched and to be thawed for about 30 minutes before issued to the patient. During the second period of the study, by June 2014, an updated guidelines were accepted to use FC products for acquired hypofibrinogenemia indications as well, FC products are considered more safe to the recipient as it is pathogen reduced, had a faster preparation time, don’t need to be ABO compatible or match and it have almost similar price to cryoprecipitate dose. Also, studies showed FC has equivalent effect as cryoprecipitate dose and it may reduce the need for allogeneic transfusion and the rate of associated transfusion reactions.15,16 The use of cryoprecipitate units reduced dramatically by more than 80% in the second period as a result of introduction of FC products as alternative therapeutic modality. Also, the implementation of near patient testing using thromboelastography (TEG®) and/or thromboelastometry (ROTEM®) in operation rooms by the second time period facilitate the use of FC inside operation rooms. Our health institution is a provisional reference for cardiac surgery, cardiac transplantation, liver transplantation, and trauma cases and therefore we may expect a high use for FC products particularly since our statistical data for PCC and FC products included products transfused for massive transfusion cases, active bleeding cases in emergency department, intraoperative cases, and cardiac surgery cases. As the increase in FC resulted from the change in use of cryoprecipitate products as a fibrinogen source (i.e. no net increase) and the fact that the use of PCC did not increase, it must be concluded that the reduction in plasma transfusion was due to the enforcement plasma transfusion policy as such and not resulting from the use of alternative coagulation factor sources for bleeding management.
Comparing the two study periods, the total number of patients admitted in the hospital and number of patient treated in the emergency department were considered equivalent (P value < 0.00001), Table 3, and therefore the reduction in plasma transfusions is expected to be due to appropriate enforcement of the policy rather than drop in number of the patients as there was no change in the clinical services provided by the hospital neither significant change in the type and number of surgical procedures performed in the two study periods.
The mortality rate for in-patients excluding mortality in operation rooms and mortality rate for patients in the emergency department excluding trauma patients were found to be not significantly different for the study periods (P value = 0.62), Table 3. The average length of hospitalization did not change significantly between the two study periods, Table 3. The study team was not able to assess patients’ morbidity that could be associated with the enforcement of the policy. However, there were no reports about excessive bleeding, complaints or complications suspected to be related to policy enforcement.
Study period |
Total number of in-patient’s admissions |
Total mortality in-patient excluding operation rooms (%) |
Average length of in-patient admission |
Total number emergency department admissions |
Total mortality in emergency department excluding trauma (%) |
1 Oct 2011 – 30 April 2014 |
65,633 |
3,201 (4.8%) |
2.6 days |
1,77,025 |
227 (0.12%) |
1 May 2014 – 30 Nov 2016 |
64,447 |
3,189 (4.9%) |
2.5 days |
1,79,609 |
237 (0.13%) |
Table 3 Comparison of total number of admissions and mortality rates for the two study periods
Unfortunately, the effect of plasma transfusions was not analyzed in this study as it was not logistically feasible. It would be of significant use to have a feedback for the clinical effect and or comparison of coagulation parameters pre- and post- plasma transfusion.
The enforcement of a policy to triage and guide appropriate plasma transfusion practice was found to be effective in significantly reducing the number of plasma transfusions by 39.5% which in order reduced time of the workload for blood transfusion services staff by 40.1%. The reduction in plasma transfusions saved 33.7% of the cost of plasma compared to the cost prior to implementation of the enforcement policy. The reported transfusion reactions associated with plasma transfusions reduced by 47.5%. The reduction in plasma transfusions did not seem to affect the mortality rate for in-patients and patients in the emergency department. In addition, there was no significant difference in the average length of hospitalization after implementing the enforcement policy. Even though the study had some limitations related to the retrospective analysis of the available data, the application of the enforcement policy is considered effective, cost-efficient and pragmatic.
The study team acknowledges Blood Transfusion Services staff, NSHA Pathology Informatics Group and Decision Support, and Health information department, at Central Zone, NSHA, for providing the required data for the study.
Authors declare that there is no conflict of interest.

©2018 Aljedani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 World Thalassemia day is observed on May 8th. Thalassemia is a genetic blood disorder that causes the body's hemoglobin level to be lower than normal, which makes you feel exhausted. This year the main motto of this day is to Empower Lives, Embracing Progress: Equitable and Accessible thalassemia treatment for all. Related submissions received for this event will be offered with 30% discount towards publication.
World Thalassemia day is observed on May 8th. Thalassemia is a genetic blood disorder that causes the body's hemoglobin level to be lower than normal, which makes you feel exhausted. This year the main motto of this day is to Empower Lives, Embracing Progress: Equitable and Accessible thalassemia treatment for all. Related submissions received for this event will be offered with 30% discount towards publication.