eISSN: 2469-2778


Case Report Volume 6 Issue 2
Department of Haematology, Cork University Hospital, Ireland
Correspondence: Vitaliy Mykytiv, Consultant Haematologist, Haematology department, Cork University hospital, Cork, Ireland, , Tel +353214922548
Received: January 23, 2018 | Published: March 23, 2018
Citation: Alwaheed A, Maraj A, Mykytiv V, et al. Secondary hemophagocytic lymphohistiocytosis in adults: a case series of 6 patients in a single institution in a brief period. Hematol Transfus Int J. 2018;6(2):66-68. DOI: 10.15406/htij.2018.06.00155
We present a case series of 5 patients of secondary hemophagocytic lympohistiocytosis (HLH) in a single institution in relatively brief period. HLH is uncommon, but it also tends to be under diagnosed, especially if it is secondary to other haematological or non‒haematological malignancy. The diagnosis is challenging and is frequently delayed. Stem cell transplantation is a potentially curative option however; it is not suitable for most patients because of age and performance status or the inability to achieve complete remission prior to transplantation. In our experience, the disease was always refractory, and the outcome was poor, regardless of the treatment that was used.
Keywords: hemophagocytic lymphohistiocytosis, fever of unknown origin, hemophagocytosis, pregnancy, chronic lymphocytic leukaemia, germ cell tumour, T‒cell lymphoma
Hemophagocytic lymphohistiocytosis (HLH) is a rare, life‒threatening disorder, with unknown real prevalence in adults. The pathogenesis is characterized by intense hyper immune response and uncontrolled inflammatory cytokine release. In addition, it involves hyper activation of macrophages, cytotoxic T lymphocytes and NK cells, leading to multi‒organ failure and eventually death. It is divided in to two main categories, familial or primary HLH and secondary HLH. Different underlying aetiologies were described to be associated with secondary HLH, with EBV infection being the commonest, followed by malignancy.1‒3
Patient characteristics and laboratory findings
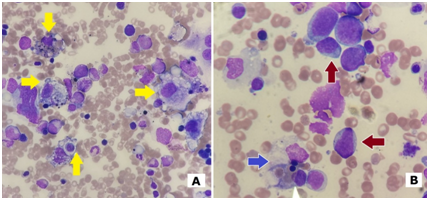
We report a series of six cases of secondary HLH in Cork University Hospital with catchment population of 620.000 during the period of 2014‒2017 (Figure 1). The diagnosis was made using HLH‒2004 criteria.4 Clinical presentation of all patients included cytopenia and high fever, initially wrongly attributed to infection. Ferritin is sensitive and accessible marker for the monitoring of disease activity however, in our experience, at presentation its levels might be only moderately elevated, achieving extremely elevated levels of >10.000µg/mL only in relapse (Figure 2). Fibrinogen and triglycerides were generally requested and detected after histological and clinical suspicion. Markers like soluble CD25, NK cytotoxicity, perforin expression are not widely available and were not performed in all patients. Sequencing of genes related with HLH was performed only in patients 1 and 2; because of the identified cause of HLH and age of the patients 3,4,5, and 6, the possibility of primary HLH was excluded. Despite of the lack of specificity, presence of hemophagocytosis in bone marrow aspirate was an inflection point in the diagnosis of all our patients.5,6 It was present in the bone marrow of patients with or without bone marrow involvement by primary cancer (Table 1). Bone marrow aspirate was usually hypocellular, and it was frequently repeated. In our opinion, if clinical suspicion of HLH is strong, phenomenon of hemopagocytosis should be investigated with perseverance by cytologist however; the final diagnosis should meet required criteria.
No infectious aetiology was identified in any of our patients. Every patient was tested for Epstein Barr virus, cytomegalovirus, human immunodeficiency virus, hepatitis A,B and C virus, human parvovirus B19. All the patients had blood culture performed and received treatment with antibiotics at some stage.
|
Patient |
1 |
2 |
3 |
4 |
5 |
6 |
|
Patient characteristics |
||||||
|
Age (years) |
33 |
48 |
65 |
33 |
63 |
75 |
|
Gender |
Female |
Male |
Male |
Male |
Female |
Male |
|
Ethnicity |
Caucasian |
Caucasian |
Caucasian |
Caucasian |
Caucasian |
Caucasian |
|
Presumed aetiology |
HELLP syndrome |
CLL on treatment with FCR |
Unknown |
Germ cell tumour |
T‒cell lymphoma |
Unknown |
|
Genetic testing for hereditary HLH† |
Negative |
Negative |
Not tested |
Not tested |
Not tested |
Not tested |
|
Abnormal perforin expression |
No |
Yes |
Not tested |
Not tested |
Not tested |
Not Tested |
|
Time to diagnosis (days) |
17 |
20 |
8 |
2 |
16 |
19 |
|
Time to 1st relapse (weeks) |
37 |
6 |
2 |
N/A |
12 |
11 |
|
Time to death (months) |
29 |
4 |
1.5 |
4 |
5.5 |
3 |
|
HLH‒2004 diagnostic criteria (at the time of diagnosis) |
||||||
|
Fever |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Splenomegaly |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Cytopenia of > 2 lines |
||||||
|
Haemoglobin, < 9.0 g/L |
7.2 |
8.8 |
9.6 |
7.9 |
8 |
6.7 |
|
Platelets <100X10^9/L |
19 |
< 10 |
<10 |
14 |
104 |
28 |
|
Neutrophils <1X10^9/L |
2.8 |
1.2 |
0.76 |
2.61 |
0.66 |
1.26 |
|
Hyperferritinemia, > 500µg/mL |
3815 |
1665 |
>15.000 |
5351 |
2244 |
2136 |
|
Ferritin in relapse, µg/mL |
>15.000 |
>15.000 |
>15.000 |
8062 |
>15.000 |
>15000 |
|
Hypofibrinogenemia or hypertriglyceridemia |
||||||
|
Fibrinogen, < 1.5 g/L |
2 |
0.9 |
1.3 |
2.2 |
0.8 |
0.6 |
|
Triglycerides, >3 mmol/L |
4.5 |
2.5 |
3.58 |
4.59 |
3.59 |
3.53 |
|
CD25s >2400 U/mL |
Not tested |
> 20.000 |
Not tested |
Not tested |
> 20.000 |
Not tested |
|
Hemophagocytosis (in bone marrow) |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Reduced or absent NK cytotoxicity‡ |
Not tested |
Normal |
Not tested |
Not tested |
Not tested |
Not tested |
|
Treatment summary |
||||||
|
1st line |
HLH 2004 protocol + CsA maintenance |
HLH 2004 protocol |
HLH 2004 protocol |
Etoposide, Cisplatin, Dexa |
CHOEP+ Dexa |
HLH 2004 protocol |
|
2nd line |
HLH 2004 protocol |
CHOEP |
N/A |
Paclitaxel, Dexa |
N/A |
N/A |
|
3rd line |
Allo‒transplant |
Etoposide, Dexa, Alemtuzumab |
N/A |
N/A |
N/A |
N/A |
|
CLL: Chronic Lymphocytic Leukaemia; FCR: Fludarabine, Cytarabine, Rituximab; HLH 2004 protocol: Dexamethasone, Etoposide, Cyclosporine; CHOEP: Cyclophosphamide, Doxorubicin, Vincristine, Etoposide, Prednisolone; CsA: Cyclosporine A; Dexa: Dexamethasone |
||||||
|
N/A: not applicable |
||||||
|
†Sequence analysis: PRF1 (FHL2), UNC13D (FHL3), STX11 (FHL4) and STXBP2 (FHL5); |
||||||
|
‡ Granule release assay |
||||||
Table 1 Bone marrow of patients with or without bone marrow involvement by primary cancer
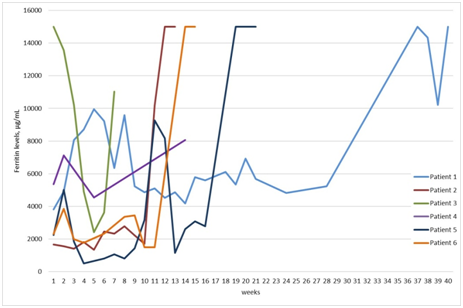
Figure 1 Ferritin levels (μg/mL) of patients 2‒5 from diagnosis to death, only the first 40 weeks are included for patient 1. Except Patient 3, who had ferritin levels > 15.000 µg/mL at the time of the diagnosis, others had only moderately elevated ferritin (< 5.000 µg/mL). Ferritin levels were significantly increased in relapse, which is seen on the graph of the Patient 1,2,3 and 6. In patient 5 there was a transient increase in ferritin levels between cycles of chemotherapy.
Underlying malignancy, as probable trigger of HLH, was identified in three of our patients. In patient 2, who was diagnosed with Chronic Lymphocytic Leukaemia (CLL), HLH could be also related to chemotherapy as he was diagnosed 10 days after third cycle of treatment with fludarabine, cyclophosphamide, rituximab (FCR). At the time of diagnosis of HLH, only 0.06% of B‒lymphocytes with CLL immunophenotype were detected by 8‒colour flow cytometry in the bone marrow sample. In addition, 50% of T lymphocytes of this patient had TCR rearrangement. Relationship between HLH and CLL treated with FCR is described in the literature.5,7
The diagnosis of the patient 1 was made in context of pregnancy and HELLP‒syndrome, and it was previously published as a single‒case report.8 Data about HELLP syndrome and HLH in the literature is sparse.
Both, germ cell tumour, presented as an extensive mediastinal mass, in case of patient 4; and T‒cell lymphoma, diagnosed on lymph node and bone marrow biopsy, in case of patient 5 are well recognised triggers of HLH.9‒11 The incidence and mortality of HLH in T‒cell lymphoma is especially high.5 No malignancy or other cause was identified in patients 3 and 6, despite multiple investigations that were performed.
Treatment and outcome
All our patients treated with HLH‒2004 protocol had a good initial response, but they have relapsed within different timeframe.4 Patients 4 and 5 were treated with chemotherapy for the primary malignancy with use of dexamethasone and etoposide. In case of Patient 5, instead of prednisolone as part of CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide, prednisolone) we use prolonged treatment with tapering dose of dexamethasone. Only patient 1 and 2 were suitable candidates for bone marrow transplant. Patient 1 received allogenic bone marrow transplant with reduced intensity conditioning still though, she relapsed 11 month later and died. Patient 2 had short term remissions and very quick relapse after his third line of treatment, therefore transplant was not possible. Patients 3 and 6 after the first relapse were unfit for further chemotherapy, and were transferred to palliative care, where shortly died. Patient 4 had another line of chemotherapy, primarily targeted at his primary cancer, before progression and death. Patient 5 has completed six cycles of modified CHOEP, because of T‒cell lymphoma as a primary cause. However, shortly after her last cycle, she had progressed, with clear clinical and biological evidence of HLH. Unfortunately, she was not fit for further treatment, and died few weeks after.
We have observed increased incidence of HLH in our institution in the period of the past two years. The fact that five of our patients have been diagnosed within the period of twelve months is alarming. In our experience, the diagnosis of HLH is challenging and frequently delayed. The course is aggressive with rapid fatal outcome without proper treatment; therefore, it often remains undiagnosed. HLH‒2004 diagnostic criteria are widely accepted however, they are not the best algorithm for adults, as they are based on small paediatric population. Morphological findings, because of its lack of specificity, are helpful only if clinical suspicion is strong. On the other hand, without morphological findings of phagocytosis the diagnosis of HLH should be made with caution. In our opinion, HLH should be included in the differential diagnosis of every patient presented with cytopaenia of more than one linage, persistent and unexplained fever, splenomegaly, and hypofibrinogenaemia. Additional markers, like lactate dehydrogenase, bilirubin or d‒dimers, can provide additional information about the activity of the disease but, they are not helpful in the differential diagnosis of HLH. If the symptoms listed above cannot be explained by an alternative diagnosis, the possibility of HLH should also be considered in the context of malignancy, which was the most common trigger in our experience. For HLH secondary to malignancy, the treatment should cover both pathologies. For that purpose, additionally to the standard of care chemotherapy, we have used dexamethasone and etoposide. However, in our experience the result was always poor, regardless of the treatment employed.
None of the authors named above received any specific grant from funding agencies in the public, commercial or non‒for‒profit sectors.
The authors declare that they have no conflict of interest.

©2018 Alwaheed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.