eISSN: 2469-2778


Research Article Volume 2 Issue 4
Haematology Department of Armed Forces Institute of Pathology, Pakistan
Correspondence: Aysha Khan, Department of Haematology, Armed Forces Institute of Pathology, CMH Road, Rawalpindi, Pakistan, Tel 923333346583, 92515517621, Fax 9251927147
Received: June 24, 2016 | Published: August 2, 2016
Citation: Khan A, Ayyub M, Ahmed S, et al. Frequency of three different gene mutations (Tel-Aml1, E2a-Pbx1 and Mll-Af4) in acute lymphoblastic leukaemia. Hematol Transfus Int J. 2016;2(4):82-85. DOI: 10.15406/htij.2016.02.00045
Background and objectives: Acute lymphoblastic leukemia (ALL) is very common in Pakistan. It is a complex genetic disease involving many fusion oncogene (FO) having prognostic significance. Its incidence may not be uniform in different parts of world because of racial, ethnic and environmental variations. These mutations have important implications for prognosis, drug selection and treatment outcome. It shows that studies on fusion oncogenes become very important for risk stratification and planning of treatment at the start of diagnosis.
Method: We studied fusion oncogene in 120 pediatric ALL patients from less than one to twenty years of age using RT-PCR and their association with age and gender. It was a cross sectional study carried out in Haematology department, Armed Forces Institute of Pathology, Rawalpindi, Pakistan over a period of one year from 1 Jan 2015 to Dec 2015. Three most common fusion genes i.eE2A-PBX1 t(1;19), TEL-AML1 t(12;21), MLL-AF4 t(4;11) were studied with relevance to their age and gender.
Results: Frequency of MLL-AF4 mutation was found in 5(4.2%) patients. TEL-AML1 was found in 13(10.8%) patients. E2A-PBX1 mutation was found in 12(10%) patients.
Conclusions: These mutations are quite frequent in our ALL patients with different age specificities. Frequencies of some of the oncogenes were different from those reported from other areas of Pakistan. These mutations are helpful in risk stratification and have therapeutic and prognostic significance.
Keywords: acute lymphoblastic leukemia, mutations, MLL-AF4, TEL-AML1, E2A-PBX1
ALL, acute lymphoblastic leukemia; FO, fusion oncogene
Acute lymphoblastic leukemia (ALL) is a neoplasm of lymphoid progenitors that may be of B or T cell lineage (B-ALL, T-ALL) and is most common malignancy of childhood.1 Its incidence varies throughout the world ranging from 0.9-4.7per 100,000 children per year.2 ALL comprises of eighty percent of childhood acute leukaemias and there is a striking incidence peak during the ages two to seven years where the incidence is as high as 10per 100,000 children.3 The outcome of ALL has improved dramatically in recent decades with cure rates exceeding 80%.4 However 20% of patients relapse, which carries poor prognosis.4 Treatment results remain unsatisfactory in adults, with a poor overall prognosis and a long term probability of survival less than 40%.Biological differences in the leukemogenesis between adult and childhood ALL may be the explanation.4
Genetic abnormalities play a major role in the prognosis and treatment outcome of ALL. It is characterized by various recurring genetic alteration including aneuploidy, structural rearrangements that commonly results in expression of chimeric fusion genes (TEL-AML1), (E2A-PBX1), (BCR-ABL) and rearrangements of MLL are important categories.5,2 Hypodiploidy and MLL are associated with poor prognosis and high risk of relapse.4,5 Different ethnic groups have different genetic mutations. ALL with TEL-AML1 also called (ETV6-RUNX1) is (12;21). It carries good prognosis responding well to the treatment. Its frequency in Pakistani population is about 17.8%.3 Some author’s claim that this translocation occur prenatally, as 1% of the cord blood cells contained this mutation which later lead to leukemia.
ALL with E2A-PBX1 Rearrangements t(1;19) In this translocation, fusion protein contains transcriptional activation domains of E2A linked to DNA binding domain of PBX1 and the encoded protein inappropriately activates the transcription of genes normally regulated by PBX1.6 Patients with this gene fusion frequently present with hypocalcaemia and coagulopathy and have poor prognosis. Its incidence also varies worldwide and in a Mexican study its incidence is reported to be 11.5%.2 Structural alterations involving band 11q23 of chromosome 11 are the frequent cytogenetic abnormality in infant ALL reaching up to 80%.5 In most cases target is MLL gene (mixed lineage leukaemia gene). ALL with MLL gene rearrangements carries bad prognosis with worst outcome in infants. Its incidence is reported to be 16.8%3 from Pakistan. Patients present with high WBC count and CNS involvement.
Treatment strategy can be planned at the beginning of diagnosis if these genetic abnormalities are known. Patients are divided into low, standard and high risk. Low risk patients are given induction therapy with regimen A, standard risk are given regimen B and poor risk patients are treated with regimen C, of UKALL 11 protocol. Intensity of the chemotherapy drugs along with their side effects increases in same order. Poor risks are than offered bone marrow transplant after induction, if they are in remission because of high risk of relapse. Treatment of other groups lasts for 2-3years.4
Objective
We conducted the study to find out frequencies of these mutations i.e. E2A-PBX1 t(1; 19), TEL-AML1 t(12; 21) and MLL-AF4 t (4; 11) in our setup.
Peripheral blood and bone marrow samples were obtained from pediatric ALL patients admitted to different hospitals of Rawalpindi and Islamabad. They belonged to different ethnic groups. Samples were collected from January 2015-2016. Patients between the ages of less than one to twenty years with confirmed diagnosis of ALL were included. These patients were newly diagnosed with no previous history of any treatment and did not have a prior severe physical illness. 120 samples were processed for molecular Cytogenetics. We studied 3 fusion oncogenes in 120 ALL patients using RT-PCR at the time of diagnosis. The clinical data were recorded at the time of diagnosis.
RNA Extraction
Total RNA was extracted from leukemic cells by TriZol LS reagent according to the manufacturer’s instruction.
Synthesis of Complementary DNA (cDNA)
RNA was reverse-transcribed to cDNA for using as template in PCR reaction. RT reaction protocol carried out briefly, 3-5μl of RNA was added to 8μl of RT mix containing, RiboLockTM RNase inhibitor, M-MuLV reverse transcriptase (Fermentas, USA), random hexamer and reaction was carried out by incubating mixture at 42°C for 60 min, 70°C for 10 min and held at 4°C in the last step.
The cDNA product was amplified with Taq polymerase. Amplication was carried out by Real time PCR in two steps, in first step there is initial denaturation, in second step there are 40 repeated cycles of denaturation, annealing & extension. Fluorescence reading was taken at 60o C step. Amplification plots were generated on ABI 7500 Real Time PCR system (Figure 1 and 2).
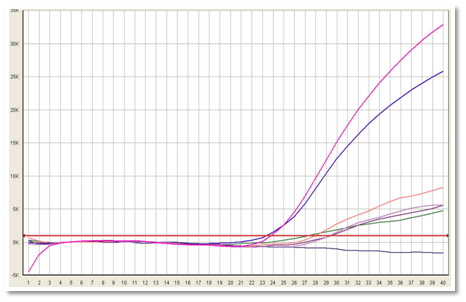
Figure 1 Positive samples were identified when fluorescence exceeds the threshold limit. Real time PCR showing MLL-AF4 gene mutation. Pink colour shows positive control, blue is patients sample while purple colour shows negative control.
Statistical Analysis
All the collected data was entered into SPSS version 20.
A total of 120 patients were included in the study. The mean age of the patients was 8.68±6.51 years and there were 68(56.7%) male and 52(43.3%) female patients in the study group. The mean hemoglobin was 8.00±1.36g/dl and the mean total leukocyte count was 180889.58±195714.63/mm3. The mean percentage of blasts was 68.44±18.82%.
MLL-AF4 mutation was found in 5(4.2%) patients. When stratified, all of the MLL-AF4 positive patients were under 2 years of age. The frequency of MLL-AF4 mutation was insignificantly higher among female patients (7.7% vs. 1.5%; p=.091) as compared to males.
TEL-AML1 was found in 13(10.8%) patients. When stratified, the frequency of TEL-AML1 was significantly higher in patients aged between 9 to 14 years. The frequency of TEL-AML1 was also higher among male patients (14.7% vs. 5.8%; p=.119) as compared to female patients but the difference was statistically insignificant.
E2A-PBX1 mutation was found in 12(10%) patients. The frequency of E2A-PBX1 was significantly higher in patients aged between 15-20 years. However, there was no statistically significant difference in the frequency of E2A-PBX1 among male (8.8% vs. 11.5%; p=.623) and female genders. These statistics are shown in Table 1, 2 and 3.
Mutations |
Frequency (n) |
Percentage |
Mean Age (Years) |
TEL-AML1 |
13 |
10.80% |
14 |
MLL-AF4 |
5 |
4.20% |
< 2 |
E2A-PBX1 |
12 |
10.05% |
15 - 20 |
Table 1 Age related frequency of ALL Mutations in Pakistan (n=120)
Fusion Oncogne |
Present study |
Awan T, et al.3 |
Siddiqui R, et al.7 |
Faiz M, et al.8 |
TEL-AML1 |
10.8 |
17.8 |
3.5 |
9.7 |
MLL-AF4 |
4.2 |
16.8 |
5 |
14 |
E2A-PBX1 |
10 |
1.9 |
0 |
2 |
Table 2 Comparison of Observed Data with Previous Studies of Pediatric ALL Conducted in Pakistan (Percentages)
TEL-AML 1 |
|
Present study |
10.20% |
Mazloumi SH, et al.9 |
5% |
Rahmani SA, et al.10 |
7% |
Chung HY, et al.11 |
17.90% |
Mesquita DR, et al.12 |
19% |
MLL-AF4 |
|
Present study |
4.20% |
Janssen JW, et al.13 |
39.90% |
Pandita A, et al.14 |
10% |
E2A-PBX1 |
|
Present study |
10% |
Pandita A, et al.14 |
5% |
Jiménez-Morales S, et al.15 |
11.50% |
Table 3 Comparison of data with international studies
ALL is a heterogonous disease and comprises of many different gene mutations. It is likely to be due to racial and geographic variations and their interactions with environment can lead to difference in their frequencies. These fusion oncogenes usually act by producing different transcriptional factors and act by different cellular pathways.16 According to genetic abnormalities, patients are divided into low, standard and high risk groups. Armed Forces Institute of Pathology is a big institute in north of the country and it receives patients from very different ethnic background, including patients from tribal areas and Peshawar which are Pathan in origin and patients from Gilgit, Baltistan and Abottabad and from north of Punjab. Most of the studies which are carried out on genetic mutations are on BCR-ABL fusion oncogene whose poor prognosis is already well established. Not much work has been done on other fusion oncogenes in this part of the country on large scale.
The mean age of the patients was 8.68±6.51 years and there were 68(56.7%) male and 52(43.3%) female patients in the study group. Iqbal16 (64.7%), Awan et al.3 (69.3%) and Faiz et al.8 (73.57%) observed similar male predominance among ALL patients in local population.3,8,16 The hemoglobin of the patients ranged from 5.2g/dl to 10.1g/dl with a mean of 8.00±1.36g/dl. Faiz et al.8 observed hemoglobin in the range of 2.3g/dl to 13.4g/dl with a median of 7.7g/dl.8 The mean total leukocyte count was 180889.58±195714.63/mm3 and the mean percentage of blasts was 68.44±18.82% in this study. MLL-AF4 mutation was found in 5(4.2%) patients. Our results match with those of another local study by Siddiqui et al.7 who observed MLL-AF4 in 5% of pediatric patients with ALL in local population.7 However much higher frequency has also been reported in local population by Awan et al.4 (16.8%) and Faiz et al.8 (14.0%).3,8 In a study by Trka et al.17 from Czech republic its incidence is 3.9%.17 In another international study its frequency was 39.9% by Janseen et al.13 Pandita et al.14 reported its frequency to be 10%.14
When stratified, all of the MLL-AF4 positive patients were under 2years of age. Awan et al.3 in 2012 however observed two peaks of MLL-AF4 first under 2years of age (29.4%) and second peak in 8-15 years of age (58.8%).16 the frequency of MLL-AF4 mutation was insignificantly higher among female patients (4:1; 7.7% vs. 1.5%; p=.091) as compared to males. A possible explanation for this apparent association can be predominance of female patients (78.13%) in that age group in the present study. As Awan et al.3 (2.4:1) and Faiz et al.8 (4:1) observed male predominance of MLL-AF4.3,8
TEL-AML1 was found in 13(10.8%) patients. Our results match with those of other local studies by Iqbal16 (10.2%). However Awan et al.3 and Iqbal et al.18 reported much higher frequency of 17.8% and 16% respectively in local population of ALL.16,18 While Faiz et al.8 (9.7%) and Faiz et al.19 (6%) reported comparatively lower frequency of TEL-AML1 mutation in local population.8,19 Among other populations Mazloumi et al.9 (5%), Rahmani et al.10 (7%) in India, Tsang et al.20 (3.3%) and Chung et al.11 (17.9%) in China, Chung et al.11 (17.1%) in Korea, Lazic et al.21 (17.1%) in Serbia and Papadhimitriou et al.22 (24.3%) in Germany reported varying frequency of TEL-AML1.9–11,20–22 Our finding supports the observation of Mesquita et al.12 that TEL-AML1 has low incidence in developing countries which may be associated with poor living standards in these countries.12
When stratified, the frequency of TEL-AML1 was significantly higher in patients aged between 9 to 14years as shown in Table 1. Our observation match with that of Faiz et al.8 who observed 90% of the patients with TEL-AML1 mutation to be aged around 10years.8 The frequency of TEL-AML1 was also higher among male patients (3.33:1; 14.7% vs. 5.8%; p=.119) as compared to female patients but the difference was statistically insignificant. Iqbal16 (2:1) and Faiz et al.8 (4:1) also observed similar male predominance among patients of TEL-AML1 mutation.8,16 E2A-PBX1 mutation was found in 12(10%) patients. Jiménez-Morales et al.15 observed a similar frequency of 11.5% in Mexican children with ALL.15 However, Faiz et al.8 observed E2A-PBX1 mutation in only 2% of patients with ALL in local population.8 Pandita et al.14 reported its frequency to be 5%.14
The frequency of E2A-PBX1 was significantly higher in patients aged between 15-20years. A similar age distribution was observed by Jiménez-Morales et al.15 However, there was no statistically significant difference in the frequency of E2A-PBX1 among male (1:1;8.8% vs. 11.5%;p=.623) and female genders. Jiménez-Morales et al.15 however observed much higher frequency of E2A-PBX1 among male patients (5:1) in Mexican patients of ALL.15 Thus mutations MLL-AF4, TEL-AML1 and E2A-PBX1 were detected in 5(4.2%), 13(10.8%) and 12(10%) patients respectively. MLL-AF4 was seen in children ≤2years of age. Frequency of TEL-AML1 was highest in 9-14years and that of E2A-PBX1 was highest in 15-20years old patients.
Though the results of our study are in line with other local studies in some aspects, yet there are considerable differences. Similar reports about ethnic differences in the disease biology, genetics and treatment outcome have been reported in adult ALL by Sabir et al.23 These differences can be attributable to population and geographical differences as hypothesized by Mesquita et al.12 Considering these differences with other populations and with the local studies with in same population, there is needed to perform a multicenter study with much larger sample size for better estimate of these mutations.
Mutations MLL-AF4, TEL-AML1 and E2A-PBX1 are quite frequent in our ALL patients with different age specificities however they have variable frequencies in different geographical areas. These mutations are helpful in risk stratification and have therapeutic and prognostic significance. In order to benefit from targeted therapy a comprehensive knowledge of these mutations should be known in every population.
None.
The author declares no conflict of interest.

©2016 Khan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.