eISSN: 2469-2778


Research Article Volume 2 Issue 3
Hospital Italiano de Buenos Aires, Argentina
Correspondence: Juan Carlos Otaso, Biochemist Group, Central Laboratory of the Hospital Italiano de Buenos Aires, Argentina
Received: May 24, 2016 | Published: June 7, 2016
Citation: Otaso JC, Martinuzzo M, Barrera L, et al. Do Pt and APTT sensitivities to factors’ deficiencies calculated by the H47-A2 2008 CLSI guideline reflect the deficiencies found in plasmas from Patient’s?. Hematol Transfus Int J. 2016;2(3):61-65. DOI: 10.15406/htij.2016.02.00040
Introduction: Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) sensitivity for detecting isolated factor deficiencies varies with different reagents and coagulometer. The Clinical and Laboratory Standards Institute (CLSI) H47A2 guideline pro-posed a method to calculate these sensitivities, but some inconsistency has been reported. This study aimed to calculate factor sensitivities using CLSI guideline and to compare them with those obtained from single factor deficient patient’s data.
Methods: Different mixtures of normal pooled and deficient plasmas were prepared (<1IU/dL to 100 IU/dL) according to the CLSI H47A2 guideline. PT with Rabbit Brain (RB) and Human Recombinant (HR) thromboplastins, APTT and factors activities was measured in an ACL TOP coagulometer. Sensitivities (maximum factor concentration that produces PT or APTT values out of the reference range) were calculated from mixtures and from patients with single factor deficiencies: 17 factor FV, 36 FVII, 19 FX, 39 FVIII, 15 FIX 15 FXI and 24 FXII.
Result: PT sensitivity with RB was as follows: FV 38 and 59, FVII 35 and 58, FX 56 and 64 IU/dL; PT sensitivity with HR was as follows: FV 39 and 45, FVII 51 and 50, FX 33 and 61IU/dL; and APTT sensitivity was as follows: FV 39 and 45, FX 32 and 38, FVIII 47 and 60, FIX 35 and 44, FXI 33 and 43, FXII 37 and 46 IU/dL, respectively.
Conclusion: Reagent coagulometer combination has adequate sensitivities to factor deficiencies according to guideline recommendations (>30IU/dL). These should not be considered as actual sensitivities because those obtained by analysing patient’s plasmas with single factor deficiencies were higher for most factors and could induce misinterpretation of the basic coagulation test results.
Keywords: APTT, coagulation factor deficiencies, thromboplastin reagent, coagulometer, phospholipid
PT, prothrombin time; APTT, activated partial thromboplastin time; CLSI, clinical and laboratory standards institute; RB, rabbit brain; HR, human recombinant; IRB, institutional review board; IL, instrumentation laboratory
Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) are the most frequently used basic coagulation tests. PT is used for extrinsic pathway factor screening in patients with a bleeding history, liver disease and disseminated intravascular coagulation, and for the control of anticoagulation with oral anti vitamin K antagonists. APTT is the test of choice to screen for deficiencies of intrinsic pathway factors VIII, IX, XI and XII. It is also used to monitor unfractioned heparin therapy, and it is one of the selected tests for lupus anti-coagulant diagnosis.1
Sensitivities of these tests to single factor deficiencies could be important when explaining alterations in a routine pre surgery evaluation or for the detection of single factor deficiencies in patients with bleeding manifestations or asymptomatic carriers of these detects, and are particularly important in hemophiliacs. Sensitivity varies according to tissue factor origin, phospholipid composition and concentration in thromboplastin reagent, as well as with the negatively charged activator, phospholipid source and concentration of APTT reagent. Additionally, the sensitivity of one particular reagent could be altered using different coagulation detection methods and instruments.
The sensitivity of reagent instrument combinations can be determined by each laboratory using plasmas from deficient patient’s2 or with mixtures of pooled normal and specific factor deficient plasmas, as it was done for APTT in some studies some time ago,3 but plasmas from patient’s with single factor deficiencies can be difficult to achieve due to the low prevalence of some deficiencies.
The Clinical and Laboratory Standards Institute (CLSI) H47A2 guideline4 proposes that the sensitivity of APTT should be studied by performing the APTT on different mixtures of commercial normal pooled plasma and deficient plasmas. It could potentially be performed in any hemostasis laboratory of median low complexity. However, more recently, it has been shown that, although this guideline is feasible,5 different results were obtained when using different deficient plasmas source, lyophilized or frozen, or lyophilized from different manufacturers.6 The objective of this study was to calculate the sensitivity of the PT and APTT, used in our laboratory, to various factor levels by following the guideline procedure and to compare the results with those obtained using plasmas from patients with a single factor deficiency.
Samples
Data from consecutive patient’s tested to diagnose, confirm or follow isolated factor deficiencies (17 factor FV, 36 FVII, 19 FX, 24 haemophilia A, 15 von Willebrand diseases, 15 Hemophilia B, 15 FXI and 24 FXII) between July 2012 and February 2015 were identified. All samples were negative for lupus anticoagulant or specific factor inhibitors. Results from 10 plasmas from healthy subjects were also included in the analyses.
The protocol was revised and approved by the Institutional Review Board (IRB) on human subject research of the Hospital Italiano de Buenos Aires Ethics Committee (CEPI). No written consent from patient’s was required because the study fulfilled the established criteria for handling discarded blood and patient’s data were collected retrospectively from the study records, identifiers were removed and the data used were anonymous.
Methods
Blood sampling: Blood was drawn by clean venepuncture into vacutainer tubes containing 1/10 volume of sodium citrated (Becton Dickinson, Franklin Lakes, NJ, USA). Platelet poor plasma was obtained by centrifugation at 200 g and room temperature for 10 min. The samples were handed according to the routine laboratory management protocol.
According to the CLSI H47A2 guideline,4 different mixtures of commercial normal pooled plasma [Normal Control Assayed, Instrumentation Laboratory (IL), Bedford, MA, USA] and deficient plasmas for each factor (IL) were prepared. Normal control plasma and deficient plasmas were reconstituted with 1mL of distilled water. Then, 10 different mixtures (1 mL final volume) were prepared at normal: deficient plasma ratios of 10: 0, 9: 1, 8: 2, 7 : 3, 6: 4, 5: 5, 4: 6, 3: 7, 2: 8, 1: 9, 0: 10 to achieve a range between <1 and 90-100IU/dL of factor activity. PT was performed with two reagents from different origin: Rabbit Brain (RB) thromboplastin (PT Fibrinogen HS+, IL) and Human Recombinant (HR) thromboplastin (HemosIL RecombiPlastin 2G, IL). APTT with silica as activator was used (APTT SP, IL). Factor activities in the mixtures were measured using one-stage coagulation assays at three dilutions. HR thromboplastin for factors V, VII and X and APTT SP for factors VIII, IX, XI and XII were used as reagents for factor activity measurement. All coagulation tests were performed in a photo optical coagulometer (ACL TOP 700, Instrumentation Laboratory, Orange burg, NY, USA).
PT, expressed as % of activity, was calibrated with HemosIL Calibration Plasma (IL) as standard with correction of the 100% activity time with the geometric mean normal prothrombin time. APTT was expressed in seconds. PT and APTT results of the mixtures were plotted against factor activities, and a nonlinear regression equation (one-phase association with a least square fit selection) was applied. Reference ranges had previously been established in our laboratory by analysing a large number (>5000) of results from previously defined healthy subjects according to the CLSI/IFCC C28-A3c guideline.7 The reference range was 70-120% for the PT and 24-40s for the APTT. Sensitivity was defined as the maximum factor concentration that produced a PT or APTT out of the reference range, <70% and >40 s, respectively.
PT, APTT and coagulation factor activity of plasmas from patients with single factor deficiencies were measured using the same methods described for the mixtures. PT and APTT sensitivities derived from patient’s results analysis were then calculated through one-phase association regression equations, as was described for lyophilized mixtures.
Best fit nonlinear regression equations obtained using lyophilized or patient’s plasma results for each factor deficiency were compared using an extra sum of F-test, which analysed if the equation obtained with each data set was different or could be described by a unique single equation. Linear regression lines and statistic calculations were performed using Graph Pad Prism 5 (Graph Pad Software, Inc. La Jolla, CA, USA).
We verified that the calculated sensitivities were only due to the effect of one deficient factor on the PT and APTT. Factor assays performed in commercial factor deficient plasmas used for the sensitivity calculation demonstrated that activities were within the normal range for all factor components except the deficient one (Table 1). Table 1 also shows coagulation factor activities within the reference range of the commercial normal pooled plasma.
Factor |
II |
V |
VII |
X |
VIII |
IX |
XI |
XII |
Normal Control Assayed |
96 |
91 |
90 |
94 |
101 |
122 |
78 |
83 |
FII Deficient |
<1 |
72 |
67 |
88 |
78 |
116 |
80 |
88 |
FV Deficient |
88 |
<1 |
88 |
88 |
78 |
114 |
83 |
69 |
FVII Deficient |
85 |
95 |
1.1 |
94 |
81 |
126 |
105 |
90 |
FX Deficient |
86 |
99 |
90 |
<1 |
68 |
106 |
89 |
78 |
FVIII Deficient |
116 |
64 |
127 |
120 |
<1 |
95 |
70 |
87 |
FIX Deficient |
111 |
121 |
107 |
112 |
111 |
<1 |
86 |
109 |
FXI Deficient |
116 |
113 |
115 |
118 |
107 |
114 |
<1 |
109 |
FXII Deficient |
111 |
104 |
135 |
110 |
107 |
114 |
93 |
<1 |
Table 1Levels of coagulation factors in commercial lyophilized pooled normal plasma and commercial deficient plasmas used to calculate sensitivities according to CLSI guideline
Results are expressed in factor activity (IU/dL) calculated using three dilutions of samples. Results in bold are those of the deficient factor on each commercial plasma used.
Factor activities in plasmas from patient’s were within the reference value for all except for the deficient factor, confirming that patient’s included in the study presented a single factor deficiency (Table 2).
Factor |
II |
V |
VII |
X |
VIII |
IX |
XI |
XII |
FV Deficient |
(n16) |
98(97104) |
93(97108) |
91(8399) |
104(100135) |
118(103133) |
92(83107) |
86(6992) |
FVII Deficient |
(n36) |
94(8697) |
97(8695) |
90(8693) |
140(98151) |
102(96124) |
88(72100) |
83(7191) |
FX Deficient |
(n19) |
97(95104) |
99(94106) |
87(8499) |
141(123147) |
111(101131) |
92(8598) |
79(7087) |
FVIII Deficient |
(n41) |
106(100115) |
91(84112) |
85(75104) |
101(91111) |
107(97114) |
84(7391) |
82(7384) |
FIX Deficient |
(n15) |
110(10119) |
99(84122) |
86(80115) |
103(88111) |
130(109135) |
91(8197) |
84(7187) |
FX Iv |
(n15) |
110(100116) |
91(87113) |
85(79106) |
97(89108) |
107(102126) |
104(99119) |
76(6982) |
FXII Deficient |
(n24) |
108(98112) |
91(87110) |
92(85108) |
99(90104) |
108(101122) |
99(92110) |
85(7890) |
Table 2 Levels of coagulation factors (excluding calculation the deficient one) in plasma from different groups of deficient patients used for sensitivity
Results are expressed in median (95% confidence interval) of factor activity (IU/dL).
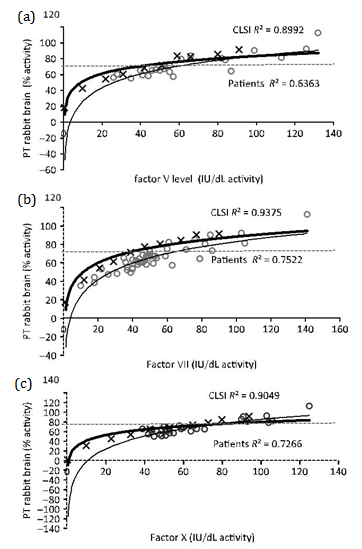
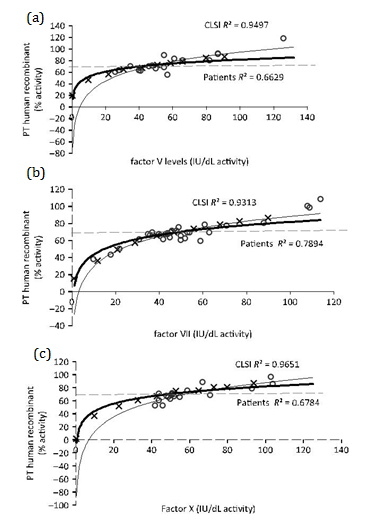
Figures 1 and 2 show the nonlinear regression lines obtained from PT measured with RB and HR, respectively, against extrinsic pathway factor levels: FV (Figure 1a & 2a), FVII (Figure 1b & 2b) and FX (Figure 1c & 2c). Lines obtained by mixtures prepared according to CLSI guidelines and patient’s plasmas are shown. Factor activities present in patient’s plasmas were 20-140 IU/dL for FV, 10-148 IU/dL for FVII and 30-138 IU/dL for FX.
Curves obtained for APTT sensitivity to intrinsic pathway factor deficiencies are shown in (Figure 3a-3d). Factor activities present in patient’s plasmas used were between <1 and 168 IU/dL for FVIII, 2 and 150 IU/dL for FIX, <1 and 117 IU/dL for FXI and <1 and 102 IU/dL for FXII.

Table 3 summarizes all sensitivities calculated by the two approaches. The differences between nonlinear regression lines and between sensitivities were statistically significant for PT RB sensitivity to FV, FVII and FX, PT HR sensitivity to FVII only and APTT sensitivity to FV, FX and FVIII. All factor sensitivities calculated for patient plasmas were similar or higher than those calculated through the guideline procedure. PT and APTT sensitivities for FII deficiency were only calculated by guideline procedure (PT RB 33 IU/dL, PT HR 35 IU/dL and APTT 20 IU/dL) because no patient’s with a single deficiency of FII were included in this study (Table 3).
Factor activity |
|||||||||||||||
Test |
II |
V |
P |
VII |
P |
X |
P |
VIII |
P |
IX |
P |
XI |
P |
XII |
P |
PT (RB) |
|||||||||||||||
CLSI |
35 |
38 |
0.008 |
35 |
0.002 |
56 |
0.012 |
– |
– |
– |
– |
||||
Patients |
– |
59 |
58 |
64 |
– |
– |
– |
– |
|||||||
PT (HR) |
|||||||||||||||
CLSI |
33 |
39 |
0.169 |
51 |
0.004 |
33 |
0.049 |
– |
– |
– |
|||||
Patients |
– |
45 |
50 |
61 |
– |
– |
– |
– |
|||||||
APTT |
|||||||||||||||
CLSI |
19 |
39 |
0.0001 |
– |
32 |
0.004 |
47 |
0.004 |
35 |
0.22 |
33 |
0.146 |
37 |
0.76 |
|
Patients |
– |
45 |
– |
38 |
60 |
44 |
43 |
46 |
|||||||
Table 3 PT and APTT sensitivities calculated according to the CLSI H47A2 guideline or using plasmas from patients with a single factor deficiency
Results of sensitivities are expressed as IU/dL of factor activity that produced PT and APTT results out of the reference range.
CLSI: Sensitivity calculated following the Clinical and Laboratory Standards Institute guideline procedure.4
Patients: Sensitivity calculated using plasma from patients with a single factor deficiency (expressed in italic). P<0.05 for extra sum of F-test comparison between nonlinear regression lines obtained by both methods. PT, APTT, RB, HR.
The CLSI guidelines have established that, ideally, the sensitivity of APTT reagent instrument combination has to be at least 30IU/dL for factors VIII, IX and XI.4 Moreover, PT and APTT within the reference values have been considered safe and used to guide transfusions in surgical interventions, assuming that factor activities were >30IU/dL.8 However, FVIII or FIX levels <60IU/dL in hemophiliac carriers have been found to increase (about two fold) the risk of bleeding manifestations, particularly after medical interventions.9 Even FXI deficiency has variable bleeding tendency, and patients with levels of 40IU/dL could present severe bleeding and low thrombin generation.10 All these suggest that it is important to recognize mild deficiencies of these factors by the most used screening test, the APTT. Furthermore, it has been reported that a particular APTT reagent instrument combination did not detect a single factor XI deficiency in a patient who bled more than expected during surgery.11 The guidelines describe procedure using commercially available pooled normal plasma and factor deficient plasmas.4 This guide procedure was considered as misleading because different sensitivities were calculated when different deficient plasmas were used, which was not explained by differences in concentrations of the other factors in them or by matrix effect of lyophilized materials compared to freeze thaw specimens, because they also showed differences when thrombin generation tests were performed on deficient plasmas.#ref6 The authors of this article recommended against following the guideline in routine laboratories and suggested that the manufacturer’s data should be used or that the sensitivities should be calculated by processing samples of patient’s with single-factor deficiencies, instead. In our study, we followed the CLSI guideline procedure4 and also calculated the sensitivities by analysing data obtained from the plasma of patient’s with a single factor deficiency and demonstrated that our APTT reagent instrument combination (micronized silica) had high sensitivity (>30IU/dL) to all factors evaluated, except prothrombin, by following the CLSI guideline.
The high APTT sensitivity obtained in our study is in concordance with that reported many years ago by Turi et al.3 who demonstrated that micronized silica reagents have good sensitivities to intrinsic pathway factor deficiencies.3 However, we demonstrated that sensitivities calculated by analysing plasmas from patients with single factor deficiencies were higher for all intrinsic pathway factors as well as for FV and FX, reaching statistically significance for FV, FX and FVIII. This is important because a mild APTT prolongation could be misinterpreted as a deficiency of factors VIII, IX or XI <35IU/dL and provoke additional studies or delay before intervention when the real factor activity is around 50IU/dL. Therefore, sensitivities calculated by the CLSI methods are artificial and could translate to inappropriate medical decisions.
We showed that the CLSI procedure could be followed to verify PT reagent instrument sensitivity to single factor deficiencies. It has been demonstrated that PT sensitivity to factors depends not only on the source of tissue factor, but also to phospholipid concentration and composition, as the presence of phosphatidyl ethanolamine increased sensitivity to FV deficiency and low concentration of phosphatidylserine decreased sensitivity to FII deficiency.12 Moreover, it has been reported that traces of FVIIa present in tissue thromboplastins and NaCl concentration affected factor deficiency sensitivities and ISI.13 Due to the variety of reagents and instruments in the market, knowledge of the sensitivity to mild factor deficiencies of the system used in the laboratory is important, in order to determine the levels needed to maintain patient’s free of bleeding. These levels have been estimated as 12IU/dL for FV, 27IU/dL for FVII, 56IU/dL for FX and 43IU/dL for combined FV+FVIII deficiency.14
In PT, HR and RB thromboplastin sensitivities calculated by following CLSI guideline were also lower than those calculated by analysing data from patients with single factor deficiencies. For both thromboplastins, sensitivities to all factors, except FII, were >30IU/dL. One weakness of our study is that patient’s plasmas were assayed at the time of blood collection for haemostasis evaluation and 3 different lots of APTT SP reagent were used during the study period. Nevertheless, the performance of each new lot of reagent was tested against the previous reagent with samples from single factor deficient, lupus anticoagulant and heparin plasmas to be sure that results were comparable. Acceptable and very consistent inter lot results (CV<5%) were observed, and also many patient’s have been studied at various times, and the results were always the same (<5% of difference).
In conclusion, we demonstrated that single factor deficiency sensitivity of PT and APTT calculated by H47A2 CLSI guideline procedure4 should not be considered as actual sensitivities, because they are lower than those obtained from fresh plasmas of single factor deficient patient’s and could lead to erroneous interpretation of basic coagulation test results. However, as this study only demonstrated this difference for a particular manufacturer reagents/instrument system with high sensitivities, it would be interesting to verify these data for other reagent instrument combinations.
None.
The author declares no conflict of interest.

©2016 Otaso, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.