eISSN: 2576-4462


Research Article Volume 7 Issue 2
1Agronomy Undergrad Student, Plant Sciences Department, Ponta Grossa State University, Brazi
2PhD Student, Ma, Plant Sciences Department, Ponta Grossa State University, Braz
3Professor, PhD., Plant Sciences Department, Ponta Grossa State University, Brazil
Correspondence: Ricardo Antonio Ayub, Professor, PhD., Plant Sciences Departament, Ponta Grossa State University, Ponta Grossa, Paraná, Brazil. Av. Carlos Cavalcanti, 4748, Zipcode: 84030-900, Tel +55 (042)21028678
Received: July 12, 2023 | Published: June 30, 2023
Citation: de Morais LM, dos Reis CA, Ayub RA. In vitro conservation of hops – Cascade and Nugget cultivar under slow-growing conditions. Horticult Int J. 2023;7(2):73-78. DOI: 10.15406/hij.2023.07.00279
Hops (Humulus lupulus L.) is economically relevant in the brewing industry. Although Brazil has a big beer market, the unfavorable climate makes the hop growing difficult. Micropropagation is an alternative way to clone plants, but in this process somaclonal subculture variations may occur. In order to reduce consecutive subcultures, the slow growth technique can be used. To develop a slow growth protocol for the conservation of hop plants and Cascade and Nugget cultivars in vitro, Murashige and Skoog (MS) medium was supplemented with doses of osmotic agents: sucrose and sorbitol. The plants were kept at 25°C or 4°C during 120 days. After 4 months, the plants were transferred to a new MS medium containing sucrose (30 g.L-1) and kept for 30 days under optimal growing conditions. After evaluation, the plants were transplanted and underwent a 30-day acclimatization period, and then the ex vitro survival was evaluated. For both cultivars, reduced growth was observed at 4°C, with a satisfactory survival rate. There was no significant interaction between the osmotic agent and temperature for Cascade cultivar Conversely, the Nugget cultivar did have interaction, and the treatment with sucrose (60 g.L-1) at 4 ºC reduced plant length, brought largest root length and fresh mass.
Hops (Humulus lupulus L.) belongs to the Cannabaceae family1 and has great economic relevance due to the use of its female inflorescence in the brewing industry. It is a dioecious species, and only the flowers of unpollinated female plants are interesting for agricultural production, due to their chemical compounds, such as alpha and beta acids and essential oils.2
Approximately 100151 hectares of hops were planted worldwide in 2020, reaching a total production of 171841 tons and a yield of 1.71 tons per hectare.2 Although the Brazilian beer market is the third-largest in the world, hop plantations are still scarce due to the unfavorable climate for the development of the plant, which needs to go through a dormancy period.2,3 This period is when the rhizomes remain under the soil and lasts from the beginning to the end of winter.4,5 By the year 2019, 48 cultivars have already been registered with Brazil's Ministry of Agriculture, Livestock, and Supply.6
Hops are generally propagated vegetatively through rhizomes or stem cuttings,7 but another option is in vitro propagation when the climatic conditions can be controlled. This methodology preserves and replicates the characteristics of the parent plant, maintaining uniformity and reducing the time of propagation.8,9 Through in vitro tissue culture, it is possible to obtain a large number of plants in a limited space, with high genetic and phytosanitary quality.10,11
The culture of plant tissues in vitro is based on the introduction of an already disinfected tissue called explant, which was obtained from the plant of origin, into a sterile and specific culture medium, generating a plant that is genetically identical to the matrix plant.8 During the cloning of plants in vitro genetic alterations such as somaclonal variations can occur as a result of consecutive subcultures.12 These alterations impose problems for the commercial micropropagation since genetic fidelity is essential.13 The slow growth technique can be used to reduce the number of consecutive subcultures, especially during winter when hops are not planted in the field. This technique consists in inducing osmotic stress, adding growth retardants, and/or decreasing the concentration of saline and organic components in the culture medium, reducing the light intensity or temperature. These events drastically bring down the metabolism of the plant without harming its viability.10,14,15
Based on the problems presented, this work aimed to find an alternative in vitro methodology to maintain the vegetative material of hops of the Cascade and Nugget cultivars in vitro for long periods without affecting the quality of specimens and with low-cost techniques.
Plant material
Two experiments were conducted to evaluate the effect of different sucrose and sorbitol concentrations and temperature on the slow growth of hop cultures. Both experiments were conducted in the Laboratory of Biotechnology Applied to Fruit Culture, at the State University of Ponta Grossa - Paraná State, Brazil.
The explants of Humulus lupulus cultivars Cascade and Nugget used in the experiment came from plants already established in vitro on MS medium16 with 30 g.L-1 of sucrose, 7 g.L-1 of agar, and 0.1 g.L-1 of inositol, which were maintained in a growth room with a 16-hour photoperiod, temperature of 25±2 ºC and photon irradiance of 40 µmol m-2 s-1.
Culture media and adaptation
The culture medium used in the experiment was MS, pH adjusted to 5.8, gelled with 7 g.L-1 of agar, and increasing concentrations of sucrose and sorbitol, which make up the treatments described in Table 1. After preparation, 30 ml of medium was transferred to glass vials and then autoclaved for 20 minutes at 121°C.
|
Treatment |
Osmotic agent |
Concentration (g.L-1) |
|
Suc30 (control) |
Sucrose |
30 |
|
Suc45 |
Sucrose |
45 |
|
Suc60 |
Sucrose |
60 |
|
Suc15+Sorb15 |
Sucrose + Sorbitol |
15 + 15 |
|
Suc30+Sorb15 |
Sucrose + Sorbitol |
30 + 15 |
|
Suc45+Sorb15 |
Sucrose + Sorbitol |
45 + 15 |
Table 1 Osmotic agent and concentration according to each treatment
Nodal segments of approximately 2 cm in length, containing a pair of axillary buds and a pair of leaves, were transferred to the experimental medium in an aseptic environment. The flasks with the plant's tissue fragments were sealed with plastic lids and transparent plastic film and were maintained in optimal culture conditions (25±2°C, 16 h photoperiod, and photon flux of 40 μmol m-2s-1).
Slow-growing conditions
After seven days under optimal culture conditions (25±2°C, 16 h photoperiod, and photon flux of 40 μmol m-2s-1), the flasks were transferred to experimental conditions of reduced temperature and light. Two storage temperatures were tested, 4 °C and 25 °C, with a 16 h photoperiod under a photon flux of 2 μmol m-2s-1, and kept for four months.
Plants submitted to the 4 °C temperature treatment were conditioned in a B.O.D (Biochemical Oxygen Demand) incubator chamber, while those subjected to the 25 °C temperature remained in a growth room. At 30-day intervals, visual evaluations of the survival (%) of the explants were performed.
Regrowth
After 120 days under the experimental conditions, the plants were transferred to a new MS medium with 30 g L-1 sucrose and then conditioned in a growth room with a 16-hour photoperiod under photon irradiance of 40 μmol m-2s-1 and the temperature of 25±2°C. 30 days after the transference to optimal cultivation conditions, the seedlings were evaluated for the length of the nodal segments (cm), the number of leaves and number of shoots, and the formation of the roots (cm) (Figure 1).

Figure 1 Hop plants of Cascade cultivar, after 5 months of in vitro culture at a temperature of 25°C and sucrose concentration at 30 g.L-1, being 120 days in a photon flux of 2 μmol m-2s-1 and 30 days in re-growth at 25°C and 2 μmol m-2s-1.
Morphometric analysis and acclimatization
Once the in vitro experimental period was over, the plants were removed from the vials and washed under running water to remove the culture medium from the roots. Excess water was removed using absorbent paper, and then the fresh weight of the plants was recorded using a precision balance. The variables number of shoots and leaves were quantified visually. The seedling length and roots was measured with the aid of a manual caliper. After evaluated, the plants were transplanted into 300 mL disposable cups containing substrate and were covered with individual plastic bags in order to maintain the high levels of humidity required for acclimatization. In each disposable cup, 5 plants were transplanted, referring to one repetition. The cups were placed on a tray permanently covered with a thin layer of water, placed near a window that allowed natural light to be captured. At the bottom of the glass, perforations were made to allow water absorption by the roots. 30 days after transplanting the plants were visually evaluated for survival (%).
Statistical analysis
In both experiments, the experimental design was entirely randomized (CRD) in a 6x2 factorial scheme (osmotic agent x temperature) with 5 repetitions per treatment and 5 plants per flask. Data were evaluated using the R Studio software (RStudio Team, 2016). The normality and homogeneity of the results were analyzed by the Shapiro-Wilk test and significant variables were compared adopting Tukey's test at 5% probability.
For the Cascade cultivar, no interaction between temperature and osmotic agent concentration was observed in any of the variables analyzed. At 25 ºC, the results for all the variables were statistically increased when the temperature was analyzed isolated (Figure 2), indicating that at higher temperatures the plants tend to develop faster. Although the plants showed reduced growth at 4 °C, when kept at 25 °C there was no need to propagate, since their average length did not exceed 5.36 cm.
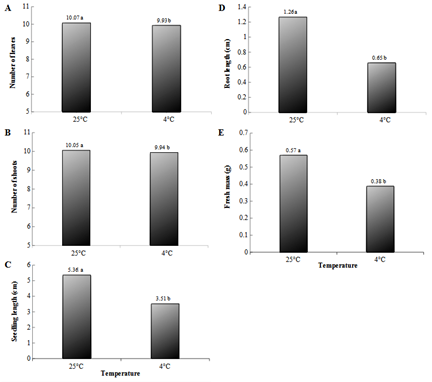
Figure 2 Number of leaves (A), Number of shoots (B), Seedling length (C), Length of the largest root (D), and Fresh mass (E) of hops plants of Cascade cultivar, maintained for 120 days under different temperatures and photon flux of 2 μmol m-2s-1 and then for another 30 days in a growth room under optimal conditions for the resumption of growth. Same letters mean no statistical difference by the Tukey test at 5% probability level.
The number of leaves was higher in the treatments sucrose (30 g.L-1) + sorbitol (15 g.L-1) and sucrose (45 g.L-1) + sorbitol (15 g.L-1) compared to other concentrations of osmotic agent tested (Figure 3A).
The number of shoots was higher in plants treated with sucrose (45 g.L-1) + sorbitol (15 g.L-1) than in plants treated with sucrose (30 g.L-1), sucrose (60 g.L-1), and sucrose (15 g.L-1) + sorbitol (15 g.L-1) (Figure 3B).
The plant lengths in the sucrose (45 g.L-1) treatment were statistically increased from sucrose (60 g.L-1) (Figure 3C). There was no need to propagate the plants during the in vitro cultivation period in any of the tested treatments because the highest length found was 5.33 cm.
The weight of the fresh mass was statistically higher in sucrose (30 g.L-1) and sucrose (45 g.L-1) groups compared to sucrose (15 g.L-1) + sorbitol (15 g.L-1) (Figure 3D).
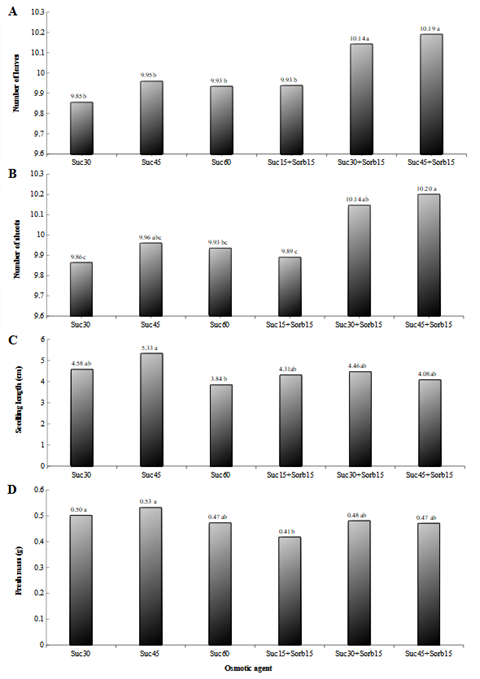
Figure 3 Number of leaves (A), Number of shoots (B), Seedling length (C), and Fresh mass (D) of hop plants of Cascade cultivar, obtained under different concentrations of osmotic agents, was maintained for 120 days under different temperatures and photon flux of 2 μmol m-2s-1 and then for another 30 days under optimal conditions for growth resumption. Treatments: Sac30- Sucrose (30 g.L-1), Sac45- Sucrose (45 g.L-1), Sac60- Sucrose (60 g.L-1), Sac25+Sorb15- Sucrose (15 g.L-1) + Sorbitol (15 g.L-1), Sac30+Sorb15- Sucrose (30 g.L-1) + Sorbitol (15 g.L-1), Sac45+Sorb15- Sucrose (45 g.L-1) + Sorbitol (15 g.L-1). Same lower case letters do not differ by Tukey's test at a 5% probability level.
During the in vitro conservation period, statistically significant differences were not observed in survival rate until 90 days. On day 120 it was possible to observe that at 25°C the survival rate was statistically superior when compared to 4°C (Table 2). During the period of ex vitro acclimatization of the plants, it was not observed any statistically significant difference between osmotic agents and temperature regarding survival rate.
|
In vitro survival (%) |
|||||
|
|
|
Days |
|||
|
Osmotic Agent |
Temperature |
30 |
60 |
90 |
120 |
|
Sucrose (30 g L-1) |
25°C |
100 ns |
100 ns |
100 ns |
100 a |
|
Sucrose (45 g L-1) |
25°C |
100 |
100 |
100 |
100 a |
|
Sucrose (60 g L-1) |
25°C |
100 |
100 |
100 |
96 ab |
|
Sucrose (15 g L-1) + Sorbitol (15 g L-1) |
25°C |
100 |
100 |
100 |
100 a |
|
Sucrose (30 g L-1) + Sorbitol (15 g L-1) |
25°C |
100 |
100 |
100 |
100 a |
|
Sucrose (45 g L-1) + Sorbitol (15 g L-1) |
25°C |
100 |
100 |
100 |
100 a |
|
Sucrose (30 g L-1) |
4°C |
100 |
100 |
100 |
100 a |
|
Sucrose (45 g L-1) |
4°C |
100 |
100 |
100 |
92 ab |
|
Sucrose (60 g L-1) |
4°C |
100 |
100 |
96 |
76 b |
|
Sucrose (15 g L-1) + Sorbitol (15 g L-1) |
4°C |
100 |
100 |
100 |
96 ab |
|
Sucrose (30 g L-1) + Sorbitol (15 g L-1) |
4°C |
100 |
96 |
96 |
92 ab |
|
Sucrose (45 g L-1) + Sorbitol (15 g L-1) |
4°C |
96 |
96 |
92 |
88 ab |
Table 2 Survival of hop plants of Cascade cultivar was maintained under different temperatures and in vitro osmotic agent fluxes of 2 μs according to storage time
*Means followed by equal lower case letters do not differ by Tukey's test at 5% probability level.
For the Nugget cultivar, in the variables "number of leaves" and "number of shoots", no significant interaction was observed between temperature and the concentrations of the tested osmotic agents. Among the temperatures, it was observed that at 25°C, both the number of leaves and the number of shoots were statistically higher when compared to the temperature of 4°C (Figure 4).
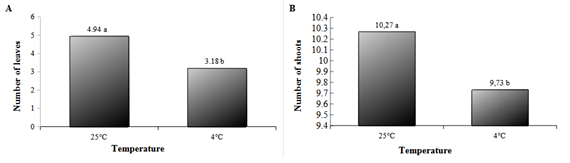
Figure 4 Number of Leaves (A) and Number of Shoots (B) of hop plants of Nugget cultivar kept for 120 days under different temperatures and photon flux of 2 μmol m-2s-1 and then for another 30 days in the growth room under optimal conditions for the resumption of growth. Same letters mean no statistical difference by Tukey's test at a 5% probability level.
For the number of shoots, the treatment sucrose (30 g.L-1) + sorbitol (15 g.L-1) was statistically different from sucrose (30 g.L-1) and sucrose (45 g.L-1), but did not differ from the treatments sucrose (60 g.L-1), sucrose (15 g.L-1) + sorbitol (15 g.L-1) and sucrose (45 g.L-1) + sorbitol (15 g.L-1) (Figure 5). The increase in sucrose concentration raised the number of shoots, however, lower sucrose concentrations associated with sorbitol, resulted in equal or higher rates of sprouting compared to sucrose alone.
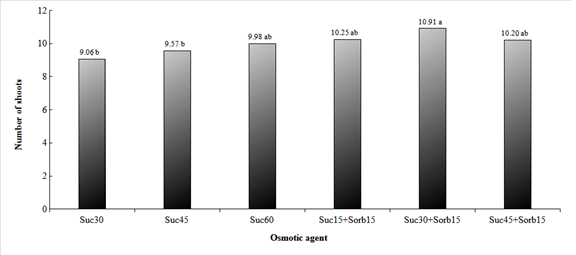
Figure 5 Number of shoots of hop plants of Nugget cultivar, obtained under different concentrations of osmotic agents, kept for days under different temperatures and photon flux of 2 μmol m-2s-1for 120, and then for another 30 days under optimal conditions for growth resumption. Treatments: Sac30- Sucrose (30 g.L-1), Sac45- Sucrose (45 g.L-1), Sac60- Sucrose (60 g.L-1), Sac25+Sorb15- Sucrose (15 g.L-1) + Sorbitol (15 g.L-1), Sac30+Sorb15- Sucrose (30 g.L-1) + Sorbitol (15 g.L-1), Sac45+Sorb15- Sucrose (45 g.L-1) + Sorbitol (15 g.L-1). Same lower case letters do not differ by Tukey's test at a 5% probability level.
For the variables “plant length” (Figure 6A), “length of the largest root” (Figure 6B), and “fresh mass” (Figure 6C), it was observed statistical interaction between the temperatures and osmotic agent levels tested.
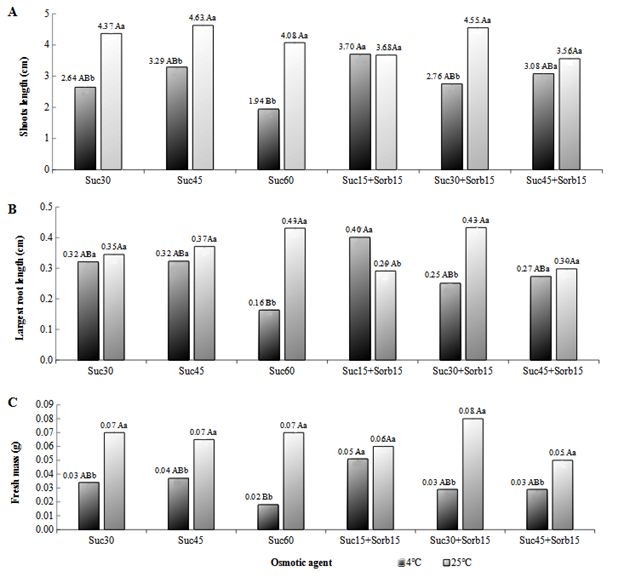
Figure 6 Plant Length (A), Largest Root Length (B) and Fresh Mass (C) of hops of Nugget cultivar, obtained under different concentrations of osmotic agents and temperatures Treatments: Sac30- Sucrose (30 g.L-1), Sac45- Sucrose (45 g.L-1), Sac60- Sucrose (60 g.L-1), Sac25+Sorb15- Sucrose (15 g.L-1) + Sorbitol (15 g.L-1), Sac30+Sorb15- Sucrose (30 g.L-1) + Sorbitol (15 g.L-1), Sac45+Sorb15- Sucrose (45 g.L-1) + Sorbitol (15 g.L-1). Equal capital letters between osmotic agent concentrations and lower case letters between temperatures do not differ by Tukey's test at 5% probability level.
For the variable “plant length”, “largest root length”, and “fresh mass”, there was no statistically significant difference between the concentrations of osmotic agents when they were kept at 25°C. When the plants were kept at 4°C, the sucrose (15 g.L-1) + sorbitol (15 g.L-1) treatment was statistically superior to sucrose (60 g.L-1) and did not differ from the other concentrations.
The fresh mass and the plant length were higher at the temperature of 25 °C compared to 4 °C at all concentrations of the osmotic agent, except for sucrose (15 g.L-1) + sorbitol (15 g.L-1) (Figure 6A and C). In the Sucrose (45 g.L-1) + Sorbitol (15 g.L-1) group, the plant length showed no statistically difference between temperatures. There was no need for seedling pricking out during the in vitro period in any of the treatments because the highest seedling length obtained was 4.62 cm.
For root length, it was observed that when sucrose (15 g.L-1) + sorbitol (15 g.L-1 ) was added to the culture medium, the length of roots formed at 4°C was statistically higher when compared to the treatment at 25°C.
During the in vitro conservation period, it was not noted any statistically significant differences in survival, during the first 30 days. On the 60th day the survival rate was statistically higher in hops kept at 25ºC compared to plants at 4°C. No statistically significant differences were seen in survival rates during the 90 and 120 days (Table 3). Although a drop in the survival rate was perceived at the time of 60 days when the plants were kept at 4°C, the lowest survival rate observed was over 90%, demonstrating that the viability was unaltered. Thus, the temperature of 4°C reduced plant development during the conservation period.
|
In vitro survival (%) |
|||||
|
|
|
Days |
|||
|
Osmotic Agent |
Temperature |
30 |
60 |
90 |
120 |
|
Sucrose (30 g L-1) |
25°C |
100 ns |
100 ns |
100 ns |
96 ns |
|
Sucrose (45 g L-1) |
25°C |
100 |
100 |
100 |
96 |
|
Sucrose (60 g L-1) |
25°C |
100 |
100 |
96 |
96 |
|
Sucrose (15 g L-1) + Sorbitol (15 g L-1) |
25°C |
100 |
100 |
100 |
96 |
|
Sucrose (30 g L-1) + Sorbitol (15 g L-1) |
25°C |
100 |
100 |
100 |
100 |
|
Sucrose (45 g L-1) + Sorbitol (15 g L-1) |
25°C |
100 |
100 |
96 |
92 |
|
Sucrose (30 g L-1) |
4°C |
100 |
100 |
96 |
96 |
|
Sucrose (45 g L-1) |
4°C |
100 |
92 |
92 |
92 |
|
Sucrose (60 g L-1) |
4°C |
96 |
96 |
96 |
96 |
|
Sucrose (15 g L-1) + Sorbitol (15 g L-1) |
4°C |
100 |
100 |
100 |
100 |
|
Sucrose (30 g L-1) + Sorbitol (15 g L-1) |
4°C |
100 |
100 |
100 |
96 |
|
Sucrose (45 g L-1) + Sorbitol (15 g L-1) |
4°C |
92 |
92 |
92 |
92 |
Table 3 Survival of hop plants of Nugget cultivar in vitro under different temperatures, osmotic agent concentrations, and photon flux of 2 μmol m-2s-1 according to storage time
*Means followed by equal lower case letters do not differ by Tukey's test at 5% probability level.
During the ex vitro acclimatization no statistically difference was observed between the osmotic agents and temperature regarding the survival rates (higher than 95.33%).
Low temperatures slow down the rate of chemical reactions that are vital to plants, and make bio membranes more rigid, requiring more energy to activate biochemical processes17 and reducing the growing, thus it is coherent to find smaller plants growing at 4ºC when compared to 25ºC, as seen in our results. Reed et al.18 in their work on cold storage and cryopreservation of hops, observed that under the conditions of 4 °C, 12-hour photoperiod, and photon flux of 3 μmol m-2s-1, plants of the Cascade cultivar remained alive for up to 21 months and the vast majority of the other cultivars tested withstood storage for longer than 12 months.
When we observe the number of leaves between treatments for the Cascade cultivar, we verify that these are more numerous in those with higher concentrations of sugars. According to Calvete et al.19 the higher content of sugars in the medium results in a higher concentration of carbohydrates in the leaf tissue, so leaves can remain longer in the plant. Possibly, the high concentration of sucrose together with sorbitol made the osmotic condition of the plant more favorable for leaf production. In relation to the number of shoots, the treatments with the lowest concentrations of sucrose presented a statistically lower values than sucrose (45 g.L-1) plus sorbitol (15 g.L-1), possibly because the plants consumed the reserve energy available in the medium more quickly, which led to a reduction of the development of shoots. Also, the higher sucrose concentration allowed it to act as an osmoregulatory agent, reducing plant growth, while the 45 g.L-1 of sucrose caused the sugar to be consumed by the plant as an energy source only, raising its growth rate.20
During the 120 days of preservation, the plants were kept in a low-light intensity environment of 2 µmol m-2s-1, and after this period, they remained for another 30 days in a photon flux of 40 µmol m-2s-1 for resumption of growth. Low light intensity possibly interfered with plant growth, slowing it down, although another experiment is needed that compares the effect of normal and low light intensity.
The fresh mass for the Cascade cultivar was higher in treatments with just sucrose when compared to sucrose plus sorbitol possibly is that hop plants lack the mechanisms for the efficient metabolization of alcoholic sugars, such as sorbitol. In response to this inefficiency, sorbitol may have acted mostly as an osmoregulatory agent, inducing the plants to consume sucrose from the medium to produce the energy required for their development.21
The Nugget cultivar did not show significant variations among sugar concentrations for the evaluated parameters at 25°C. These differences in physiological responses between cultivars still need to be investigated.
The reduction in plant growth may have been caused by the low light intensity to which they were exposed throughout the 120 days of storage. According to Ballester et al.22 and Reed et al.,18 the use of low light intensity (3 to 8 μmol m-2s-1) was effective in reducing plant metabolism.
Possibly, sorbitol and low sucrose concentration triggered reactions in the plant metabolism in response to modifications in osmotic concentrations; together, these results protected the roots from cold, since alterations in osmotic conditions can improve the protection of plant tissues from disturbances in metabolic pathways.23,24
Even though the in vitro survival rate was lower at low temperatures, the plants resumed their development satisfactorily after the conservation period.
The reduction of in vitro growth of hop plants was more efficient at 4°C, both for the cultivar Cascade and for Nugget. In both situations, there was no need to subculture the plants during the 120 days that they were kept stored. Sorbitol did not reduce plant growth. Regardless of the cultivar, the treatment that was most effective in reducing the growth of hop plants and extending the period between subdivisions was the concentration of 60 g.L-1 of sucrose associated with a temperature of 4º.
None.
None.

©2023 de, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.