eISSN: 2469-2794


Research Article Volume 12 Issue 1
Technical-Scientific Police Superintendence of the Goiás State, Brazil
Correspondence: Advaldo Carlos Souza Neto, Instituto de Criminalística Leonardo Rodrigues, Avenida Engenheiro Atílio Correia Lima n. 1223 - Cidade Jardim, Goiânia, Goiás, Brazil
Received: January 16, 2024 | Published: February 2, 2024
Citation: Neto ACS, Pimentel KN, Bezerra LSA, et al. Detection sensibility and amplification threshold establishment: Internal validation of Investigator® Quantiplex® PRO in a genetic forensic laboratory in central Brazil. Forensic Res Criminol Int J. 2024;12(1):25‒28. DOI: 10.15406/frcij.2024.12.00394
The present study aims to describe the internal validation of DNA quantification process in the Forensic Biology and DNA Laboratory (LBDF) of Goiás, Brazil. The sensitivity, precision and accuracy of the Investigator® Quantiplex® Pro, a real-time human DNA quantification kit, were evaluated. We used three blood samples with known genetic profile and tested the parameters in triplicate with DNA concentration ranging from 1ng/µl to 0.0005ng/µl. We observed that the kit has high sensibility, detecting all samples. The precision and accuracy of the Y-chromosome target was lower comparing with the small and large autosomal targets. The threshold of 0.005ng/µl was established to proceed to DNA amplification. The internal validation conducted provided parameters for decision-making regarding the amount of human DNA required for amplification in the laboratory.
Keywords: DNA quantification, quality assurance, real time PCR, threshold, validation, Quantiplex®
PCR, polymerase chain reaction; RIBPG, Integrated Network of Genetic Profile Databases; LBDF, Forensic Biology and DNA Laboratory
Forensic genetics is a scientific discipline that utilizes molecular biology techniques for criminal investigation and the identification of missing persons.1 Within this field, an increasingly crucial tool is DNA profile databases. These databases facilitate the exchange of data among different laboratories, with the goal of identifying the perpetrators of crimes and establishing human identification. In Brazil, these databases are interconnected through Integrated Network of Genetic Profile Databases (RIBPG), coordinated by a multidisciplinary Steering Committee.2 The advancement of forensic genetics and the increasing of DNA databases in investigative process over recent years have necessitated the establishment of quality management system requirements. These requirements are crucial to guarantee the reliability and accuracy of results produced by the laboratory.3 It is widely recommended, including in Brazil,4–7 that laboratories undergo accreditation to the ISO/IEC 170258 standard. Among the ISO/IEC 17025 process requirements, method validations are essential to cater to the specific needs of a particular field of application.8
Among the analytical methods that must be validated in a forensic genetics’ laboratory is DNA quantification.9 DNA quantification is a pivotal process in forensic genetics, particularly for samples collected from crime scenes, which commonly exhibit low DNA quantity, degradation, and the potential presence of PCR inhibitors. Furthermore, DNA quantification is a critical step in the decision-making process for forensic experts, influencing whether to proceed with the analysis.10,11 Various DNA quantification methods exist, but real-time PCR is commonly used in laboratories due to its high sensibility and specificity. Among the available commercial kits is the Investigator® Quantiplex® Pro (QIAGEN®). This kit enables the determination of human DNA quantity, assessment of DNA degradation levels, identification of the presence of PCR inhibitors, and the evaluation of the ratio of male to female DNA in the sample.12 In this context, the present study aimed to perform the internal validation of the Investigator® Quantiplex® Pro (QIAGEN®) kit. The validation aimed to assess the sensitivity of human DNA detection and establish a threshold for the detected DNA quantity in the sample sufficient for subsequent amplification. This validation was carried out in the Forensic Biology and DNA Laboratory (LBDF) of the Technical-Scientific Police Superintendence of the Goiás State, Brazil.
DNA extraction
DNA extraction was conducted on three blood samples with a known genetic profile (one male and two females). The extraction process was performed in triplicate using the PrepFiler Express™ Forensic DNA Extraction Kit (Applied Biosystems®) on AutoMate Express™ DNA Extraction System (Applied Biosystems®) method.
DNA quantification
The extracted DNA underwent real-time PCR quantification on the Applied Biosystems™ 7500 Real Time PCR System using the Investigator® Quantiplex® Pro (QIAGEN®) kit. Subsequently, the samples (n= 9) were diluted to the following concentrations: 1ng/µL, 0.5ng/µL, 0.1ng/µL, 0.01ng/µL, 0.005ng/µL, 0.001ng/µL and 0.0005ng/µL. These solutions were then quantified again to evaluate the sensibility and accuracy of the Investigator® Quantiplex® Pro (QIAGEN®) kit.
Precision and accuracy evaluation
For precision assessment, the mean, standard deviation, and coefficient of variation of each replicate at every concentration were calculated. Accuracy was evaluated by determining the percentage error relative to the expected concentrations. Statistical analysis, including ANOVA and Tukey Test, was performed to assess the mean error of each group.
Amplification DNA threshold
To establish the amplification DNA threshold, all solutions were amplified using the PowerPlex® Fusion 6C amplification kit (PROMEGA®). Additionally, the male samples were also amplified using PowerPlex® Y23 amplification kit. The amount of DNA from each concentration present in the amplification reaction is detailed in Table 1. The percentage of amplified alleles was evaluated to infer the amplification threshold.
|
Dilution point |
Concentration (DNA) |
DNA available in PCR reaction |
|
1 |
1 ng/µl |
1 ng |
|
2 |
0.5 ng/µl |
0.5 ng |
|
3 |
0.1 ng/µl |
0.5 ng |
|
4 |
0.01 ng/µl |
0.15 ng |
|
5 |
0.005 ng/µl |
0.075 ng |
|
6 |
0.001 ng/µl |
0.015 ng |
|
7 |
0.0005 ng/µl |
0.0075 ng |
Table 1 Amount of DNA available on amplification reaction
Sensitivity
The Investigator® Quantiplex® Pro kit (QIAGEN) demonstrated remarkable sensitivity as it successfully detected all samples, affirming its capability to identify even low quantities of DNA (≥0,0005 ng).
Precision
The results obtained from quantification using both the small and large target exhibited lower standard deviation and coefficient of variation to those observed in the Y chromosome target (Table 2). This suggests that the results pertaining to the quantity of male DNA in the sample are comparatively more imprecise.
Accuracy
According to accuracy, the error percentage was calculated by comparing the results to the expected concentration. Notably, for the autosomal target (small), significant differences were observed in mean error of 0.001ng/µl and 0.0005ng/µl in comparison to all other concentrations (ANOVA, F= 6.152, p <0.0001 – Tukey test). The errors at these concentrations were significantly larger than those at other concentrations, indicating that the kit has a loss in accuracy in low DNA concentrations (Table 2, Figure 1). In contrast, such pattern was not observed for the other targets. However, a higher percentage error was noted for the Y chromosome target, indicating not only higher imprecision but also greater inaccuracy for this particulate target (Table 2, Figure 1). This underscores the importance of considering potential accuracy limitations, particularly in the context of low DNA concentrations and the specific Y chromosome target.
|
Expected DNA Concentration |
Mean concentration observed |
Coefficient of variation |
Error Percentage |
|
1 ng/µL (Small) |
1.0077±0.1377 (n=9) |
13.66 |
12.22±4.74 |
|
0.5 ng/µL (Small) |
0.5174±0.0574 (n=9) |
11.09 |
9.4±6.79 |
|
0.1 ng/µL (Small) |
0.1032±0.0153 (n=9) |
14.83 |
13.01±7.47 |
|
0.01 ng/µL (Small) |
0.0108±0.0022 (n=9) |
20.37 |
17.13±14.63 |
|
0.005 ng/µL (Small) |
0.0055±0.0005 (n=9) |
9.09 |
11.45±6.43 |
|
0.001 ng/µL (Small) |
0.0012±0.0003 (n=9) |
25 |
31.62*±23.95 |
|
0.0005 ng/µL (Small) |
0.0008±0.0003 (n=9) |
37.5 |
57.77*±48.03 |
|
1 ng/µL (Large) |
1.0267±0.1273 (n=9) |
12.4 |
9.39±8.42 |
|
0.5 ng/µL (Large) |
0.5373±0.0627 (n=9) |
11.67 |
10.42±9.89 |
|
0.1 ng/µL (Large) |
0.1077±0.0150 (n=9) |
13.93 |
14.24±7.90 |
|
0.01 ng/µL (Large) |
0.0116±0.0021 (n=9) |
18.1 |
19.39±17.05 |
|
0.005 ng/µL (Large) |
0.0058±0.0007 (n=9) |
12.01 |
17.79±12.52 |
|
0.001 ng/µL (Large) |
0.0012±0.0003 (n=9) |
25 |
22.09±21.80 |
|
0.0005 ng/µL (Large) |
0.0005±0.0003 (n=9) |
60 |
34.86±39.27 |
|
1 ng/µL (Y) |
1.2312±0.2815 (n=3) |
22.86 |
27.41±21.62 |
|
0.5 ng/µL (Y) |
0.5913±0.1327 (n=3) |
20.92 |
18.26±26.54 |
|
0.1 ng/µL (Y) |
0.1204±0.0186 (n=3) |
15.45 |
20.44±18.60 |
|
0.01 ng/µL (Y) |
0.0122±0.0043 (n=3) |
35.25 |
32.27±32.41 |
|
0.005 ng/µL (Y) |
0.0049±0.0016 (n=3) |
32.63 |
25.46±7.35 |
|
0.001 ng/µL (Y) |
0.0015±0.0005 (n=3) |
33.33 |
47.29±50.45 |
|
0.0005 ng/µL (Y) |
0.0012±0.0018 (n=3) |
150 |
237.49±283.87 |
Table 2 Precision and accuracy of all targets in all sample concentrations of Investigator® Quantiplex® Pro kit
*Values statistically different from other small target categories (ANOVA p <0.0001, Tukey Test)
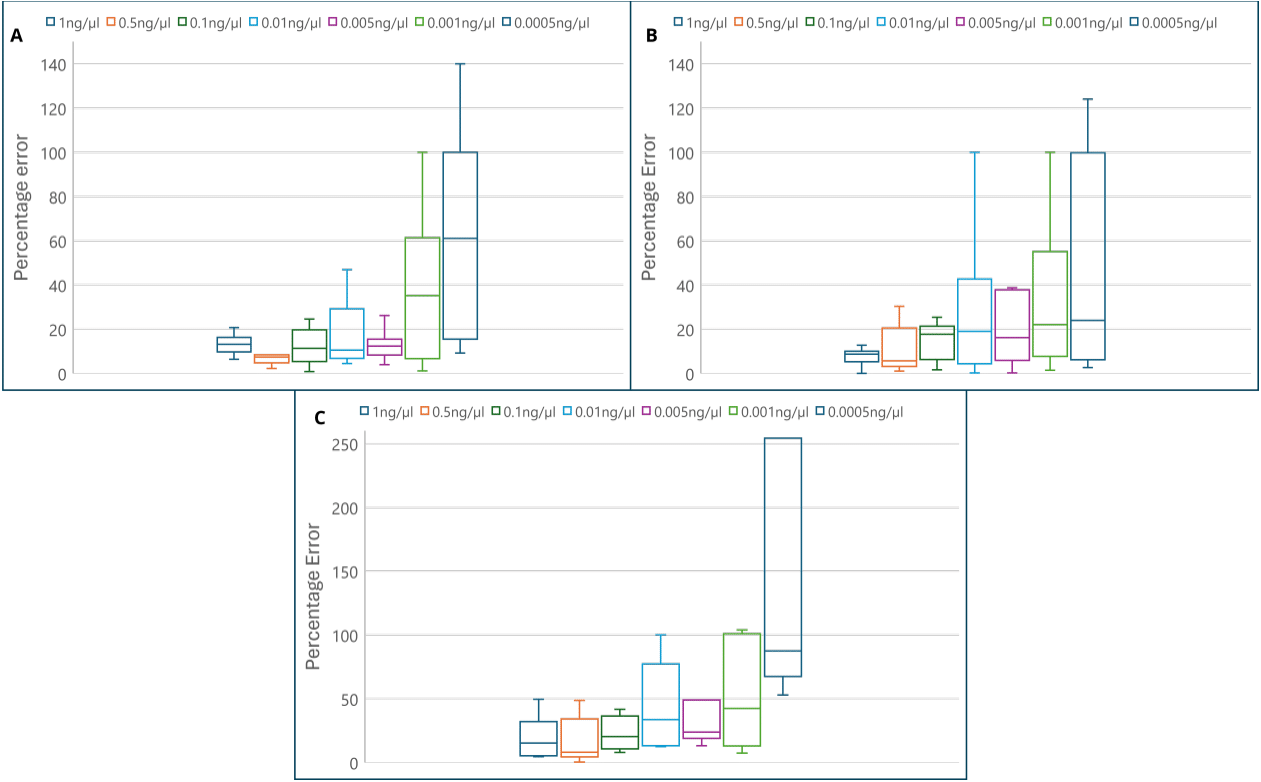
Figure 1 Percentage error for the three targets of Investigator® Quantiplex® Pro. A – Autosomal (small); B – Degradation (large); C – Y chromosome.
Amplification threshold
To establish an amplification threshold, the samples were amplified using autosomal (PowerPlex® Fusion 6C) and Y chromosome (PowerPlex® Y23 – for male samples) amplification kits. All samples with concentration equal to or above 0.005ng/µl (equivalent to 0.015ng of DNA in the amplification reaction) were successfully amplified. In these instances, all alleles were present in the electropherograms, displaying high allele height (RFU) as indicated in Table 3.
|
Sample DNA concentration |
DNA quantity on PCR reaction |
Mean percentage of amplified alleles (Autosomal) |
Mean height (RFU) of amplified alleles (Autosomal) |
|
1 ng/µL |
1 ng |
100±0 (n=9) |
7159.54±1798.3 (n=9) |
|
0.5 ng/µL |
0.5 ng |
100±0 (n=9) |
7568.51±1114.10 (n=9) |
|
0.1 ng/µL |
0.5 ng |
100±0 (n=9) |
6939.44±886.97 (n=9) |
|
0.01 ng/µL |
0.15 ng |
100±0 (n=9) |
1901.67±388.36 (n=9) |
|
0.005 ng/µL |
0.075 ng |
100±0 (n=9) |
871.68±148.37 (n=9) |
|
0.001 ng/µL |
0.015 ng |
47.78±12.82 (n=9) |
280.68±29.20 (n=9) |
|
0.0005 ng/µL |
0.0075 ng |
10.88±6.52 (n=9) |
239.17±23.62 (n=9) |
|
Sample DNA concentration |
DNA quantity on PCR reaction |
Mean percentage of amplified alleles (Y chromosome) |
Mean height (RFU) of amplified alleles (Y chromosome) |
|
1 ng/µL |
1 ng |
100±0 (n=3) |
8681.22±5427.35 (n=3) |
|
0.5 ng/µL |
0.5 ng |
100±0 (n=3) |
10018.20±568.04 (n=3) |
|
0.1 ng/µL |
0.5 ng |
100±0 (n=3) |
9561.44±1663.65 (n=3) |
|
0.01 ng/µL |
0.15 ng |
100±0 (n=3) |
1916.96±517.62 (n=3) |
|
0.005 ng/µL |
0.075 ng |
100±0 (n=3) |
806.55±133.44 (n=3) |
|
0.001 ng/µL |
0.015 ng |
14.49±15.27 (n=3) |
201.25±176.93 (n=3) |
|
0.0005 ng/µL |
0.0075 ng |
1.45±2.51 (n=3) |
69±119.51 (n=3) |
Table 3 Amplification success of samples with different concentrations
However, samples with lower concentrations exhibited a significant loss of alleles. Consequently, based on these findings, the amplification threshold established for LBDF was determined to be 0.005ng/µl. This threshold ensures reliable and consistent amplification outcomes, particularly crucial when dealing with samples of lower DNA concentrations.
The validation process assumes pivotal importance in enhancing the objectivity of responses provided by forensics experts, especially when dealing with samples characterized by low DNA quantities. Our results align with the findings of Vraneš et al.,12 who concluded that Investigator® Quantiplex® Pro kit (QIAGEN) exhibits high sensitivity to the DNA presence in samples. This heightened sensibility is especially important in forensic scenarios, such as sexual assault cases. In instances of sexual assault investigations, the ability to detect male DNA in the victims’ body, as facilitated by high sensitivity kits like Investigator® Quantiplex® Pro, is of significant relevance. This detection, when coupled with medical forensic examination, serves as a key indicator of sexual intercourse.
The validation for DNA quantification holds significant importance, as underscored by recommendations from various committees.13–15 This process represents a pivotal stage in DNA forensic analysis, crucial to ensuring the accuracy and reliability of subsequent genetic profiling. As highlighted by Gonzales et al.,9 validation plays a key role in establishing the required DNA quantity thresholds for proceeding with amplification process. Our results indicate that samples with concentrations above 0.005ng/µl are deemed suitable for amplification, both for autosomal and Y chromosome kits. This established threshold serves as a valuable tool for forensic experts in decision-making, guiding them in obtaining interpretable genetic profiles. Furthermore, adopting this threshold enables the laboratory to optimize processes and reduce costs, as samples falling below the specified threshold are excluded from the PCR process. This strategic approach enhances efficiency and resource utilization in forensic DNA analysis.
This study has inherent limitations, primarily stemming from the fact that the analyzed samples were devoid of degradation signs. As a result, variations in response may be anticipated depending on the quality of the forensic samples subjected to analysis. In the presence of degradation and/or interfering agents, the amplification response tends to decrease, potentially rendering the established threshold inadequate for achieving a complete genetic profile. Acknowledging these limitations, it is imperative for laboratories to identify sources of uncertainty and ensure their clear definition within the management system. Moreover, as a crucial next step, we recommend conducting validation studies specifically focused on DNA amplification. Extending these analyses to encompass other equipment and kits available in the laboratory is also advisable. This broader validation scopes enhance the reliability and applicability of the findings across a spectrum of forensic scenarios, accounting for potential variations introduced by diverse sample qualities and investigative conditions.
This study has successfully completed the validation studies for the DNA quantification process utilizing the Investigator® Quantiplex® Pro kit (QIAGEN) within a Brazilian forensic analysis laboratory. Through a comprehensive analysis, the study evaluated detection sensitivity, precision, and efficiency. The resulting findings not only contribute to optimizing processes and reducing costs but also enhance overall efficiency and resource utilization in forensic DNA analysis.
The authors express thanks to the team of the Forensic Biology and DNA Laboratory of the State of Goiás, Brazil, whose contribution was essential in the execution of actions.
The authors declare there is no conflict of interest.

©2024 Neto, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.