eISSN: 2473-0815


Research Article Volume 12 Issue 4
1Department of Endocrinology, KPC Medical College and Hospital, India
2Community Medicine, Medical College and Hospital, India
3Department of Endocrinology, Vivekananda Institute of Medical Sciences, India
Correspondence: Anirban Majumder, Professor, KPC Medical College and Hospital, Kolkata, India, Tel +91 9830078837
Received: November 29, 2024 | Published: December 27, 2024
Citation: Majumder A, Mukherjee P, Chaudhuri SR, et al. Tailored feminising hormone therapy and breast development: a real-life experience in a referral center cohort of Eastern India.Endocrinol Metab Int J. 2024;12(4):140-145. DOI: 10.15406/emij.2024.12.00360
Individuals with gender incongruence seeking gender affirmation as female (transgender women) may pursue hormonal and/or surgical gender-affirming interventions. Breast augmentation, the surgical procedure, though available, is associated with complications and prohibitive costs. Several studies have demonstrated the efficacy of estrogen to induce feminisation in transgender women, but the same in Indian transgender women was never evaluated. Here we retrospectively examined the breast development that occurred with feminising hormone therapy within a cohort of 50 adult transgender women in India. The relative contribution of age, body mass index (BMI), weight change with oral estrogen therapy, addiction status, estradiol levels, and GnRH therapy on breast development was also analyzed by Statistical Package for Social Sciences (SPSS) version 23. Significant breast development was noted in all the groups of patients with variable duration of estrogen treatment compared to baseline. Sustained and continuous development was observed with continuity of estrogen therapy even after 3 years.
Keywords: gender incongruence, transgender women, feminising hormone therapy, estrogen therapy, breast development
Gender incongruence is defined as an incongruence of one’s gender identity with the sex assigned at birth. Management of a person’s gender incongruence aims to affirm the individual’s gender identity through a combination approach that includes counseling, gender-affirming hormone therapy, and sometimes gender-affirming surgeries to reduce dysphoria.1 Persons diagnosed with gender incongruence and seeking gender affirmation as female (transgender women or male-to-female women), are treated with feminising hormone therapy in order to enable them to better align their physical and/or psychological features with feminine phenotype.2 Estrogen and anti-androgen, the gender-affirming hormone therapy, leads to the feminisation.1 Hormonal regimens for transgender women are not standardized across the world. Adjustments to estrogen regimens are usually guided by a given patient’s phenotypic response to therapy. Breast development is a key feature of feminisation and is the most important consideration to transgender women.
Literature regarding how much development can be expected from estrogen alone is extremely sparse and inconsistent and the available evidence suggests that breast development is insufficient for the majority of transgender women with estrogen therapy.2S Moreover, because of the anatomical differences between cis-women (female sex assigned at birth matches with gender identity) and transgender women, breast size may appear smaller than the actual size when objectively measured in transgender women populations.3 Consequently, many transgender women seek additional breast augmentation besides estrogen therapy.4,5 A small section of women with breast augmentation surgery have capsular contracture or lead to re-operations due to complications,6 and many transgender women are unable to obtain surgeries owing to prohibitive costs. The relationship between estrogen therapy in Indian transgender women and breast development has not been investigated. It is important to have clear expectations for the extent and timing of breast development with feminising hormone therapy to be able to reduce the number of breast augmentation surgeries often associated with complications, and prohibitive costs.
This study aims to examine the absolute breast development in Indian transgender women during gender-affirming hormone therapy retrospectively. Furthermore, the relative influence of clinical (age, baseline body mass index [BMI], weight change with estrogen, addiction, and concomitant gonadotropin releasing hormone [GnRH] analogues therapy) and laboratory (serum estradiol) parameters on breast development have also been explored in this study.
Data of adult gender incongruent people 18 years or older, diagnosis confirmed by a psychiatrist, seeking gender affirmation as female, and receiving gender-affirming hormone therapy in the outpatient department of endocrinology of a tertiary care hospital (K P C Medical College & Hospital) of Eastern India were evaluated retrospectively. The department followed a standard protocol laid down by the Endocrine Society1 and the recently published consensus statement from India.7 Feminising hormone therapy for the cohort comprised of administration of estradiol valerate combined with antiandrogens (spironolactone with or without finasteride) and/or with GnRH, with due consideration of cost and adverse reaction.
Those received feminising hormone therapy between January 2017 and December 2021 and whose baseline and follow-up measurements of breast and chest circumference and laboratory parameters were available included for analysis.
Those received feminising hormone therapy before 2017, underwent breast augmentation surgery, or gonadectomy or received irregular feminising hormone therapy (assessed by multi-item survey instrument, Morisky Medication Adherence Scale)8 or lost to follow-up were excluded from data analysis.
Height (centimeter), weight (kilograms), BMI (kg/meter2), breast circumference (centimeter), chest circumference (centimeter) and addiction habit (smoking/alcohol/others) were routinely captured at baseline and all follow up visits in the out-patient medical record manually. Measurement of breast circumference was done with a measuring tape horizontally placed round the thoracic cage over the bare breasts at its fullest part. Circumference of the chest was measured with the measuring tape in a horizontal line in the inframammary fold (Figure 1). Both measurements were done in standing posture with breath held in expiration. The difference between breast circumference and chest circumference (breast-chest difference) were also routinely calculated in each follow-up visit to assess the impact of feminising hormone therapy on breast development.
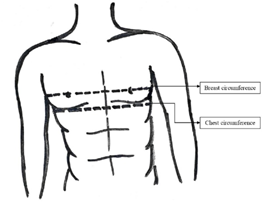
Figure 1 Measurement of breast and chest circumference
A measuring tape was horizontally placed around the thorax over the fullest part of the bare breasts to measure
Breast circumference. A measuring tape was horizontally placed around the thorax in the inframammary fold to measure the chest circumference.
The study protocol was approved by the Institutional Ethics Committee. This retrospective cohort study was conducted as ethical consideration prevented us from a randomized, placebo-controlled study (with no estrogen in control group) following the published consensus guidelines.7 Confidentiality and anonymity were maintained. In accordance with the Endocrine Society,1 all transgender women were advised to attend the outpatient clinic every 3 months, but many missed scheduled appointments. Data were captured as per the real-life follow-ups in the outpatient clinic and so the data of all patients for every visit were not available for analysis. All data including laboratory parameters were collected retrospectively from the out-patient medical record.
Serum estradiol was drawn at baseline, and every three months of estrogen therapy as routine care in the department. Quantitative determination of 17-β estradiol was carried out using Cobas e 411 electrochemiluminescence immunoassay autoanalyser supplied by Roche Diagnostics. Estradiol assay employed a test principle with two monoclonal antibodies specifically directed against 17-β estradiol. Endogenous estradiol released from the sample by mesterolone competes with the added estradiol derivative which was labeled with a ruthenium complex, binded sites on the biotinylated antibody. The result of the assay was confirmed via a calibration curve which was generated by 2-point calibration. Precision within the assay series was evaluated by processing ten serum replicates. For estradiol, the result was a mean 94.3 ± 5.8 (interassay coefficient of variation, CV 4.7%) and a lower limit of quantitation (LOQ) of 5.00 pg/ml.
Data were first entered in MS Excel Office 10 version. Statistical package for Social Sciences (SPSS) version 23 was used to analyses the data. When categorical variables were considered percentages and frequencies were calculated and for parametric continuous variables baseline data were presented as mean with standard deviation, and median with range for nonparametric data. Tobacco usage and alcohol usage data, concomitant GnRH therapy and prior feminising hormone therapy were presented as percentage of users. Kruskal Wallis test was used to see the association of breast development with duration of estrogen therapy and other factors. For test of difference between paired groups, Wilcoxon Signed Ranked test was used.
Total 50 transgender women were included in the study at baseline for analysis. The quantitative variables (age, height, weight, BMI, breast and chest circumference, breast-chest difference, oral estrogen dose and serum estradiol level) were analyzed for normality of distribution by Kolmogorov-Smirnov test and except age, breast-chest difference, oral estrogen dose and serum estradiol level (P<0.05) all other variables were found to be normally distributed.
The clinical parameters (age, height, weight, BMI, addiction habit [smoking/alcohol/others], concomitant GnRH therapy, concomitant antiandrogen therapy & gender affirming hormone use before baseline visit), breast measurements (breast circumference, chest circumference and breast-chest difference) and laboratory parameter (serum estradiol) at baseline were represented in Table 1 (n=50). The median age was 26 years (range, 18 to 43 years) of the cohort at baseline. The mean breast-chest difference was 3.61±2.93 cm, median 2 cm. All transgender women received antiandrogens and only 6 transgender women received concomitant GnRH analogue therapy along with oral estrogen valerate. Eleven transgender women, out of a total of 50, used hormone therapy before the first visit.
|
Parameters |
Value * |
|
Age (years) (median, range) |
26 (18-43) |
|
Height(cm) (mean ± S.D) |
165.50±6.35 |
|
Weight (kg) (mean ±S.D) |
61.79±11.22 |
|
BMI (kg/m2) (mean ±S.D) |
22.67±3.72 |
|
Smoking, n (%) |
19(38.00) |
|
Alcohol use, n (%) |
25(50.00) |
|
Other addiction, n (%) |
0(0) |
|
Concomitant GnRH therapy, n (%) |
6(12) |
|
Concomitant Antiandrogen therapy, n (%) |
50 (100) |
|
Gender affirming hormone use before baseline visit, n (%) |
11(22) |
|
Breast circumference, cm (mean ±S.D) |
82.47±9.62 |
|
Chest circumference, cm (mean ±S.D) |
78.86±8.80 |
|
Breast-chest difference, cm (median, range) |
2(0-11) |
|
Serum estradiol levels (pg/ml)(median, range) |
34.09 (4-440) |
Table 1 Clinical, breast measurement and laboratory parameters at baseline (n=50)
Table 2 depicts the number of transgender women in five categories according to duration of estrogen exposure; 0 months or baseline (Group A), 3-6 months (who attended 1st follow-up, Group B), 7-12 months (who attended 2nd follow-up, Group C), 13-24 months (who attended 3rd follow-up, Group D), 25-36 months (who attended 4th follow-up, Group E) and 37-60 months (who attended 5th follow-up, Group F). Some transgender women missed certain follow-up visits. Major number (n=48, 96%) belonged to the group-C who attended 2nd follow up and sufficient in number (n=40, 80%) belonged to the group-D who had attended 3rd follow up of estrogen exposure. Group D had sufficiently long estrogen exposure [attended both 2nd follow-up & 3rd follow-up] and the data analyzed further in Table 5 & 6.
|
Group |
Duration of estrogen therapy in months |
Patients attended the clinic n [ %] |
|
|
Group A |
0 months (Baseline) |
50 [100] |
|
|
Group B |
3-6 m (1st follow-up) |
30 [60] |
|
|
Group C |
7-12 m (2nd follow-up) |
48 [96] |
|
|
Group D |
13-24 m (3rd follow-up) |
40 [80] |
|
|
Group E |
25-36 m (4th follow-up) |
20 [40] |
|
|
Group F |
37-60 m (5th follow-up) |
7 [14] |
|
Table 2 Categorization of patients with respect to the duration of estrogen therapy
Breast development occurred in all the groups: 0.60 cm in Group B, 1.19 cm in Group C, 3.16 cm in Group D, 5.18 cm in Group E and 8.14 cm in Group F after estrogen treatment. With continuation of estrogen therapy breast development continued. There was statistically significant difference of breast development between groups with variable duration of estrogen therapy (p=0.000)-vide Table 3.
|
Group |
Meanbreast-chest difference at baseline (cm) [a] |
Mean breast-chest difference at follow-up (cm) [b] |
Mean breast development (cm) [b-a] |
H statistic(df) |
p-value† |
|
|
Group B[n1=30] |
3.18 |
3.78 |
0.6 |
47.617(4) |
p=0.000 |
|
|
Group C [n2=48] |
3.57 |
4.76 |
1.19 |
|||
|
Group D[n3=40] |
3.04 |
6.2 |
3.16 |
|||
|
Group E [n4=20] |
3.35 |
8.53 |
5.18 |
|||
|
Group F [n5=7] |
3.14 |
11.29 |
8.15 |
|||
Table 3 Comparison of breast development between groups with different duration of estrogen therapy
†p calculated by Kruskal Wallis test, p<0.05 considered as statistically significant
Table 4 showed pair wise comparison by post hoc Kruskal Wallis test. Breast development was non-significant between data of Group B & data of Group C, between data of Group D & data of Group E and between data of Group E & data of Group F (adjusted p=1.00, 0.395, 1.00 respectively). Breast development was significant between Group C & Group D (adjusted p=0.012).
|
Group |
Duration of estrogen therapy in months |
Test statistic |
Adjusted p value¶ |
|
Group B vs Group C |
3-6m vs 6-12m |
-10.5 |
1 |
|
Group C vs Group D |
6-12m vs12-24m |
-28.67 |
0.012 |
|
Group D vs Group E |
12-24m vs 24-36m |
-23.28 |
0.395 |
|
Group E vs Group F |
24-36m vs 36-60m |
-11.14 |
1 |
Table 4 Pairwise comparison of groups showing association of breast development with duration of estrogen therapy
¶ p computed by post hoc Kruskal Wallis test, p<0.05 considered as statistically significant
Table 5 represented the difference in breast development with duration of sufficiently long estrogen exposure for Group D (n=40) who attended both 2nd follow-up & 3rd follow-up. It was observed that compared to 2nd follow-up, breast development was more significant (p=0.000) in 3rd follow up in these patients.
|
At baseline |
6-12m (2nd follow-up) |
12-24m (3rd follow-up) |
Z statistic |
p value‡ |
|||
|
Mean breast-chest difference (cm) [c] |
Mean breast-chest difference (cm) [d] |
Mean breast development [d-c] |
Mean Breast-chest difference (cm) [e] |
Mean breast Development [e-c] |
|||
|
3.04 |
4.09 |
1.05 |
6.2 |
3.16 |
-4.268 |
0 |
|
Table 5 Breast development with duration of estrogen therapy for patients (Group D, n3=40) who attended both 2nd follow-up & 3rd follow-up
Table 6 represented the analysis of Group D (n=40) to evaluate the influence of other factors on breast development. No significant variation (p>0.05) was found in breast development between different categories of age, BMI, weight changes during oral estrogen therapy, addiction (smoking and/or alcohol), the serum estradiol level at baseline and concomitant GnRH therapy.
|
Factors |
Categories |
n (%) |
Mean Rank |
H statistic |
df |
p-value§ |
|
Age |
18-22y |
11 (27.5) |
17.5 |
1.51 |
3 |
0.68 |
|
23-27 y |
12 (30.0) |
20 |
||||
|
28-40y |
16 (40.0) |
23 |
||||
|
41-60y |
1 (2.5) |
19.5 |
||||
|
BMI at baseline |
18 to 25 kg/m2 |
30 (75.0) |
20.42 |
2.61 |
2 |
0.27 |
|
25.1 to 29.9 kg/m2 |
9 (22.5) |
18.78 |
||||
|
>30 kg/m2 |
1 (2.5) |
38.5 |
||||
|
Weight changes during oral estrogen therapy |
Weight loss |
15 (37.5) |
19.6 |
0.351 |
2 |
0.84 |
|
No weight change |
6 (15) |
22.92 |
||||
|
Weight gain |
19 (47.5) |
20.45 |
||||
|
Addiction |
Smokers |
27 (67.5) |
22.04 |
1.46 |
1 |
0.23 |
|
Non-smokers |
13 (32.5) |
17.31 |
||||
|
Alcohol |
20 (50.0) |
20.55 |
0.001 |
1 |
0.978 |
|
|
Non-alcohol |
20 (50.0) |
20.45 |
||||
|
Serum estradiol at baseline |
First quartile (0-30 pg/ml) |
12 (30.0) |
23.67 |
3.008 |
3 |
0.39 |
|
Second quartile (30.1-50 pg/ml) |
16 (40.0) |
21.56 |
||||
|
Third quartile (50.1-100 pg/ml) |
11 (27.5) |
15.59 |
||||
|
Fourth quartile (>100 pg/ml) |
1 (2.5) |
19.5 |
||||
|
GnRH therapy |
Yes |
5 (12.5) |
15.6 |
1.02 |
1 |
0.312 |
|
No |
35 (87.5) |
21.2 |
Table 6 Association of factors other than duration of estrogen therapy influencing breast development in Group D (n3=40)
Though the sense of feminisation has not been quantified, transgender women are primarily interested in sufficient degree of breast development from the gender affirming hormone therapy.2 Female breast development and growth happens: first before birth (hormone-independent), then at puberty, and later during the childbearing years, mostly in response to surging estrogen and progesterone levels. The breast consists of two tissue types, glandular (with ducts and lobules) and adipose connective tissue.9 Progesterone stimulates the formation of the glandular tissue and estrogen stimulates the formation of adipose connective tissue. Breast development with estrogen administration in transgender women is not similar to that of cis-women as substantial amount of androgens are present in transgender women (with intact gonads), inhibit mammary epithelial proliferation and breast growth.10 This may contribute to the suboptimal breast development (less than an AAA bra-cup size) in transgender women with feminising hormone therapy.11 The interactions between exogenous estrogen exposure and endogenous androgens on transgender women breast development are not studied. Supplementation with supraphysiological dose of estrogen reduces testosterone concentration because of the impact of negative feedback on the hypothalamic-pituitary-gonadal axis but is insufficient to suppress the levels into the normal range for females.1 Addition of antiandrogen medications (GnRH) are often used to further inhibit testosterone production or to further block the androgen receptor (spironolactone with or without finasteride).1 Furthermore, measurement of the chest circumferences which were used for estimation of breast development in the current cohort, proved to be a less than reliable indicator when we compared an actual breast development with the measurement of volumes.12
According to previous studies, breast development was expected to begin 3-6 months after the initiation of estrogen therapy and the maximal effect was expected after 2 years of continuous therapy.1,13 Another study suggested that main breast development occurred during first 6 months of therapy with no further raise after one year.11 Unfortunately, data is very scare with regards to the effects of feminising hormone therapies on the breast development in transgender women. Transgender women in this cohort had experienced substantial breast development with continuous estrogen therapy (estrogen valerate 1 to 6 mg daily) combined with antiandrogens (100 to 200 mg daily spironolactone with or without 5 mg daily finasteride) and/or GnRH (Triptorelin acetate 3.75 mg prolonged-release depot formulation once in every 4 weeks given deep IM). The adjustments of estrogen dose in our cohort were guided by a given patient’s phenotypic response, and also by the serum levels of estradiol. The mean estradiol valerate dose started at the baseline was 1.36 mg (± 0.48) for 50 transgender women and gradually up-titrated to a mean dose of 2.87 mg (± 1.24) after 13-24 months (40 patients). The mean dose was further up titrated to 4 mg (± 0.0) among the 7 patients who continued follow up for 37-60 months.
It also showed that breast development started after 3-6 months of estrogen treatment (0.60 cm in Group B), continued with increasing duration of estrogen therapy (Group C, D, E) and reached 8.15 cm after 37-60 months of estrogen therapy (Group F). This emphasized on uninterrupted continuity in therapy to achieve the maximum benefit. Though breast development occurred in all the groups with estrogen treatment, statistically significant difference existed between Group C & Group D (adjusted p=0.012) when pair wise comparison between the groups were done by post hoc Kruskal Wallis test.
It reflected a bit slow growth in 1st follow-up (Group B) and 2nd follow up (Group C), statistically significant growth between 2nd (Group C) & 3rd follow up (Group D); but again slowed down between 3rd (Group D) & 4th follow-up (Group E) and also between 4th (Group E) & 5th follow up (Group F). However, sustained and continuous development was evident with continuity of therapy even after 3 years. Significant progression in breast development over 2 years of treatment of estrogens and antiandrogens was similarly observed in other studies as well.1,14 However, genetic variation might play a role for the difference in breast development between different studies with different ethnic populations. 15,16
The mean age of transgender-identification and first time presentation was less than 16 years in many Western studies.17 But the mean age of presentation was quite late (about 26 years) in our country due to lack of social and informative support.4 Median age (26 years) in the current cohort remained the same with an age range between 18-43 years. No significant variation in breast development was observed between different categories of age.
Transgender women with higher BMI seemed to have more pronounced breast development with estrogen therapy. Hence, it was suggested that slender transgender women should not make much efforts to avoid the modest estrogen-induced gain in weight.13,15 Most of the patients (75%) in the current cohort had normal (18 to ≤25 kg/m2) baseline BMI and the current study found no significant variation in development of breast growth between different baseline categories of BMI that may be attributed to the small number of overweight (9 persons) and obese (1 person) in the cohort under study.
Clinically, estrogen therapy increases bodyweight (1–3 kg per year) and after 1 year of estrogen therapy fat mass (both visceral and subcutaneous) increases (2–4 kg) and lean body mass decreases (2–4 kg).18 Change in body weight with estrogen therapy was also observed in our cohort. Interestingly, 37.5% transgender women had reduction in bodyweight with estrogen therapy, possibly due to continuous emphasis on lifestyle modification in our center. No significant variation in breast development was observed between different categories of weight changed during oral estrogen therapy in our cohort.
Although drug abuse (marijuana or intravenous drug) among transgender individuals were commonly reported in Western studies, only smoking and/or alcohol was reported among Indian transgender population.19 Thirty eight percent of transgender women were smoker and 50% were alcohol users in our current cohort. No significant variation in breast development was observed between presence or absence of addiction.
Consuming oral contraceptives among Indian transgender women without medical advice in an attempt to feminise their appearance was commonly observed and a significant proportion (16%) even underwent prior castration before attending the Endocrine clinic for feminising hormone therapy.4 Out of total 50, eleven patients who used hormone therapy before first visit, mostly used estrogen either alone or in combination with desogestrel/norgestrel/finasteride, (mostly in the form of oral contraceptive pills) and a single patient had used finasteride in combination with spironolactone. Exposure to such exogenous gender affirming hormone therapy from self-medication with various formulations of oral contraceptives (11 patients in this cohort) gave rise to wide fluctuation in the basal estradiol level from 4-440 pg/ml. However, no significant variation in breast development was observed between different categories of serum estradiol levels at baseline.
Concomitant GnRH analog therapy was used in few transgender women (12%) as adjunctive medication to effectively reduce the serum testosterone level.1,7 But the use of GnRH analogs was limited in India mainly because of its high costs.5 Concomitant GnRH analog therapy, although utilized by very small number of patients, had no significant influence in breast development of this cohort.
This study provided a valuable insight in to the breast development that could be expected from gender affirming hormone therapy among Indian transgender women. No predictive markers either clinical or laboratory derived (baseline serum estradiol levels) were found in this respect. However, the study had some limitations as well. First, it had a small sample size and a retrospective in nature. Second, breast development was not measured with bra cup sizes as done in other studies,11 and neither breast volume was calculated. Instead, the difference between breast circumference and chest circumference (breast-chest difference) were used for estimating breast development, which possibly a less reliable metric compared to volume measurements. Third, this retrospective study had captured the real data from the patients of regular care and thus it was difficult to collect the cent percent data due to incomplete patients follow up as expected in most real world studies. Lastly, this study only looked at objective outcomes of breast development and did not look into the satisfaction index of transgender women with regards to the breast development that was attained finally.
In conclusion, this single-center cohort study among Indian transgender women on gender affirming hormone therapy showed significant breast development with feminising hormone therapy and the development continues even after 3 years with continuity of therapy. No predictive markers in clinical (age, baseline BMI, weight change with estrogen therapy, addiction, concomitant GnRH therapy) and laboratory parameter (baseline serum estradiol levels) were identified. Future multicenter prospective studies are needed with larger sample size, using breast volume measurements and incorporating body and/or breast satisfaction indices to relate the clinically observed development of the breast tissue to the satisfaction that the transgender women themselves perceived out of it.
We would like to express our deepest appreciation to Ms Soma Chakraborty for generously providing her knowledge, expertise and support.
None.

©2024 Majumder, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.