eISSN: 2473-0815


Research Article Volume 10 Issue 1
University of Science Arts and Technology, Montserrat, British West Indies, the University of Health and Humanities,Virgin Islands (UK), and the Einstein Medical Institute, NPB, FL, USA
Correspondence: Dr Orien L Tulp, University of Science Arts and Technology, Montserrat, British West Indies, the University of Health and Humanities,Virgin Islands (UK), and the Einstein Medical Institute, NPB, FL, USA
Received: April 12, 2022 | Published: April 25, 2022
Citation: Tulp OL. Sympathetic blockade on diet induced thermogenesis in the congenic LA/Ntul//-cp rat. Endocrinol Metab Int J. 2022;9(3):14-19 DOI: 10.15406/emij.2022.10.00313
The effects of overfeeding highly palatable diets via the cafeteria feeding diet method (Café) has been shown to be a reliable experimental model to induce diet induced thermogenesis (DIT) in young adult normally lean rats of several strains. Groups of young adult lean LA/Ntul//-cp rats were offered a Purina chow diet (CHOW) or the same diet plus a daily café supplement both ad libitum from 10 until 24 weeks of age. The drug α-methylparatyrosine (α-MPT) was administered to groups (250 mg α-MPT/kg BW, i.p.) to ablate sympathetic (SNS) activity or a sham injection of physiologic saline given, and measures of fasting resting thermogenesis obtained at thermal neutrality (30°C) before and after the α-MPT or sham administration. The Café diet resulted in a 67% increase in body weight (BW) and a ~25% increase in resting oxygen consumption (VO2) following café overfeeding in both groups, while only a 40% increase in BW and no additional increase in VO2 occurred in normally CHOW fed rats while the sham injection was without any variations from normal physiologic responses as predicted. Sympathetic blockade with α-MPT was associated with modest decreases in body temperature and an average ~12-15% decrease in VO2 in the café treated group, but when the VO2 data were arithmetically corrected to isothermal conditions only a net 3% decrease occurred in CHOW fed rats and was without effect on the SHAM group. Serum T3 concentrations increased by 92% and urinary catecholamine excretion of VMA >250% following the café diet, but the urinary vanilmandelic acid (VMA) excretion was virtually nil following the sympathetic blockade. These observations indicate that the SNS-mediated contribution component to DIT following prolonged café overfeeding under conditions of thermal neutrality contribute approximately 50% of the thermic response. While under normal long term CHOW feeding the SNS component may be as little as 3% of the thermic response, and thus, the total thermic response of DIT likely represents a combination of short acting SNS and longer acting non-SNS mechanisms, including a likely significant thyroidal component in normally lean animals.
Keywords: Thermogenesis, sympathetic ablation, α-methylparatyrosine, rats, overfeeding
Alterations in the expression of thermic responses to alterations in diet and environment are normal physiologic mechanisms of many non-human species including rodents and have also been demonstrated to occur in humans.1–6 In addition, the capacity to undergo changes in energy expenditure during periods of caloric excess and to conserve non-essential energy expenditure during periods or energy restriction are a likely survival attribute to warm blooded species.2,7,8 The physiologic and biochemical mechanisms that contribute to the regulation of energy homeostasis during variations in diet and environment are complex and remain incompletely elucidated, and clearly involve both hormonally mediated components and metabolic processes of multiple organ systems. Several authors have noted similarities in the expression of diet-induced thermogenesis (DIT) and cold-induced thermogenesis (CIT), but the physiologic mechanisms which an animal undergoes during their differential expression remain incompletely defined, although both diet- and cold-induced mechanisms both appear to invoke an activation of thermogenic activity of brown adipose tissue (BAT).9 Both processes likely have contributions from other potentially thermogenic tissues and hormonal actions including liver and skeletal muscle via direct and indirect actions.2 The multisystem contributions to thermic responses to diet and environment enable the activation of both acute and longer lasting responses.
The congenic LA/N//-cp rat was derived from the Lister Albany rat (LA), noted for its normal metabolism and long lifespan at the small animal genetics laboratory at the Veterinary Resources Branch of the National Institutes of Health (NIH) and the -cp trait derived from the Koletsy rat followed by 12 cycles of backcrossing to attain congenic status by Hansen.10 The lean phenotype of this strain has been observed to remain healthy for 48 months or more in our laboratory under conventional housing conditions.2
Acute thermogenic responses are modulated predominantly via the neurohormone norepinephrine secreted predominantly by the sympathetic component of the autonomic nervous system in response to both dietary and environmental stimuli.11 The noradrenergic responses enable an organism to elicit a rapid response to accommodate short term thermogenic demands of diet and environment, and act via activation of thermogenesis in BAT in man and animals. The BAT contains a robust blood and nerve supply in addition to an abundant supply of specialized mitochondria that are tailored for the rapid mobilization of fatty acids from multiple small lipid locules, followed by oxidative conversion of ATP to ADP, resulting in the generation of heat which may be consumed in the maintenance of body temperature or dissipated as heat when generated in abundance.9,12,13 The duration of noradrenergic responses is linked to membrane receptor activity and typically may last for minutes to hours, until the neurohormone has undergone inactivation via conversion to inactive metabolites and the metabolic processes stimulated by the neurohormonal activation have subsided. In contrast, longer term thermic responses involve expression of intracellular metabolic activities, often induced by nuclear receptor binding to sites that are highly specific hormonal receptors, and which thermogenic effects may persist for days or weeks following activation, and which likely contribute a substantial control of mechanism of energy expenditure during episodes of over- and under-nutrition. A principal hormone in the long-term regulation of energy metabolism rests with the thyroidal contributions, which are modulated via inner or outer ring deiodination via a T4-5 deiodinase enzyme or T4-5’ deiodinases to generate inactive vs active forms of triiodothyronine (T3) respectively during period of under – or over nutrition. Deiodination of T4 to T3 occurs via tissue specific D-1 or D-2 deiodinase, while inactivation of the T4 prohormone to reverse T3 (rT3) occurs via D-3 deiodinase. The nuclear receptors that modulate numerous energy- generating pathways are highly specific for T3 formed via D-1 or D-2 deiodinases and act in concert with other hormonal entities including adrenal glucocorticoid actions which can attenuate actions of other hormonal entities including insulin.14,15 Thus, the overall regulation of cellular energy metabolism and expenditure is hormonally complex and multifactorial. The cumulative effects of the sympathetic nervous system (SNS) and other contributors may be conveniently quantified via measures of resting and noradrenergic-mediated oxygen consumption (VO2), which demonstrate a close correlation between cellular and whole animal metabolism. Lean rats of this and other strains have been shown to result in increased DIT when overfed, including enhanced thermic responses to catecholamine administration accompanied with elevations in circulating T3 but not T4 concentration. In addition, these thermogenic responses have often been demonstrated to be impaired in obese animals of this and other strains.2,16
The impaired thermic responses to adrenergic stimulation have been linked to insulin resistance, which commonly accompanies the obese state in man and animals.1,4,5 The relationship between the SNS and thyroidal responses is unclear however, as is the relative proportions each physiologic response that may contribute to the thermogenic responses in both the short and long term. Early overnutrition has been shown to increase the mass and cellularity of the brown adipose tissue, and the increased BAT mass once formed is presumed to remain present thereafter, implying long term potential residual thermic capacity and a greater capacity for dietary and environmental induced changes in energy expenditure to may occur. Variations in basal metabolic rates (BMR) due to alterations in thyroidal activity are well established, with increasing T3 and BMR during periods of caloric excess and decreases in T3 and conservation of energy expenditure during periods of caloric deprivation, and including the switching from conversion of the prohormone T4 to its metabolically more active form T3 or decreased activity or conversion of T4 to its metabolically inactive form rT3 during caloric restriction. Therefore, these represent well-established implications for thyroid hormones to elicit changes in resting metabolic rates and resulting biochemical mechanisms that contribute to energy homeostasis.17 The relative contributions of the SNS and non-SNS systems to energy homeostasis continue to remain unclear, however.
The drug α-methylparatyrosine (α-MPT) can chemically ablate SNS activity by inhibition of tyrosine hydroxylase, well established as the rate limiting enzyme of catecholamine neurotransmitter biosynthesis.17–19
During the characterization of the α-MPT, Widerlov demonstrated that following a loading dose the brain content of norepinephrine and dopamine decreased to 10 to 13 % of controls with 24 hours and remained diminished for at least 32-40 hours post-treatment.20,21 Because of the profound inhibitory effect on tyrosine hydroxylase on ablation of SNS actions, α-MPT has often been used to clarify a number of nutritional and environmentally induced adrenergic activities in brain, BAT, and other tissues, and thus can facilitate the estimation of non-SNS contributions to DIT, NST, and CIT, and is without effect on thyroidal hormone formation.21
The purpose of the present study was to determine the relative contributions of SNS and non-SNS contributors to NST in chronically café overfed and in normally fed lean rats. The lean phenotype of the LA/Ntul//-cp strain typically remains quite lean throughout its lifespan and has been shown to elicit a significant thermogenic response to the café diet and cold-induced stimuli. It was deemed of interest therefor to determine the relative proportions contributed by both thermogenically- mediated physiologic systems.2–4
Eighteen young lean male littermate LA/Ntul//-cp rats were selected from our breeding colony and maintained on Purina chow #5001 from weaning at 4 weeks of age in plexiglass housing lined with one inch of pine shavings at 20°C and 50% relative humidity. Animals were randomly assigned to one of three treatment groups of 6 rats/treatment group and fed the Purina chow diet (CHOW), or the CHOW plus a cafeteria (café) diet supplement consisting of assorted carbohydrate snacks, a 10% sucrose solution in place of normal tap water and numerous palatable food items until 24 weeks of age. The third groups of rats received the same CHOW diet and house water to form the SHAM control group. Body weights of rats were monitored throughout the study. At 24 weeks of age, measures of resting VO2 were obtained in SHAM, Control, and café groups before and 24 hours after administration of α-MPT methyl ester (1.02 mM/kg BW, i.p., deemed equivalent to 250 mg/kg BW) or a SHAM injection of an equal volume of pH 7.4 Physiologic saline. The drug and SHAM injections were not associated with any untoward or unpredicted effects. The café diet was fed from 8 until 20 weeks of age and urine was collected for determination of Vanilmandelic acid (VMA) following the α-MPT administration to assess the effectiveness of the SNS ablation and is not known to impact on circulating T3 concentrations within the time frame being studied. The rats were sacrificed by decapitation under light pentobarbital anesthesia at the end of the study. Measure of circulating T3 were determined by solid phase radioimmunoassay and data were analyzed by paired t-test and student Neuman-Keuls subgroup analysis via standard statistical procedures. Measures of VO2 were determined under thermal neutral conditions (30°C) established for rodents, corrected to surface area as described by Kleiber and Wang et al.22 with a Collins small animal respirometer apparatus and plexiglass small animal chamber specially modified to enable strict temperature control.23 The measures of thermogenesis were further corrected to account for potential α-MPT induced differences in body temperature due to decreases in SNS activity at the time of measurement.24 Animals of all groups were maintained in the same facility in adjacent cages to ensure that all animals were subjected to the same environmental conditions throughout the study. Data were analyzed by ANOVA Student-Neuman- Keuls subset analysis to identify differences in outcome within the different treatment groups. This study was approved by the Biomedical Ethics and the Institutional Animal Care and Use Committees prior to the conduct of the study.
The body weights of the three groups are shown in Table 1 below and show that the initial body weights of the three groups were similar at the onset of the study, but that the Café group gained significantly more weight than the normally fed groups by the completion of the study, averaging a net gain of over 46g compared to Chow fed animals. Administration of the α-MPT resulted in modestly significant decreases in BW in both control and café fed animals, but was without effect in the SHAM group, which received an equal volume of 0.90% saline in place of the α-MPT treatment.
Live Body Weight in grams/rat |
|||||
Group |
N |
Initial BW |
Final BW |
Net Gain in BW |
g.BW Change w/α-MPT |
Control |
6 |
222± 9a |
312±12a |
90± 4a |
-3±1b |
Café |
6 |
221±10a |
360± 7b |
139± 4b |
-6±1b |
Sham Control |
6* |
228±11a |
320±12a |
92± 6a |
+3±1a |
ANOVA |
|
NS |
<0.05 |
<0.05 |
<0.05 |
Table 1 Body weights and weight gain of rats
Data are mean±1 SEM, N= 6 rats/treatment group, Initial body weights at 10 weeks of age, final BW at 24 weeks of age except where indicated. Letters after mean values indicate Neuman-Keuls subgroups at p=<0.05 level of significance. [*] BW of sham was 12 weeks of age at onset and 26 weeks of age at end of study. All measures taken after an 8 hour fast
Because the ablation of SNS activity was predicted to result in a decrease in body temperature in normally fed and housed rats, even while being maintained in a normal laboratory habitat, measures of colonic temperature were obtained before and 24 hours after the α-MPT treatment as summarized above. Body temperature values in all groups were within the normal range before the α-MPT treatment but were modestly decreased in both groups 24 hours after being administered the α-MPT treatment. In contrast, the SHAM treatment was without effect on the percent change in colonic temperature.
Blood was collected at the end of the study in fasted animals, and measures of serum triiodothyronine (T3) and 24-hour urine excretion of the catecholamine metabolite vanilmandelic acid (VMA) determined. As depicted in Figure 1A, serum T3 was significantly increased in the café fed animals compared to the normally fed animals, consistent with earlier reports of nutritional-induced increases in circulating T3 in the lean phenotype of this rodent and other rodent strains.2,6 The café dietary regimen resulted in highly significant increases in serum T3 concentrations (Figure 1A) and equally significant increases in 24-hour urinary VMA excretion (Figure 1B).
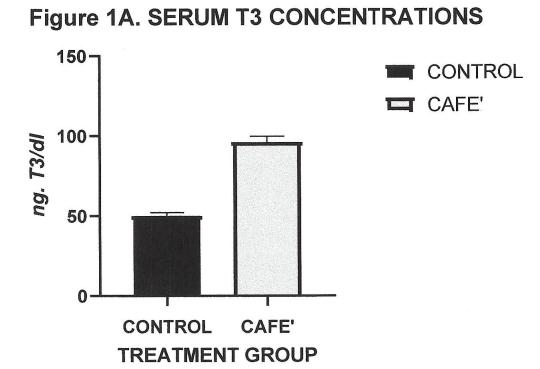
Figure 1A Effect of Café Diet on serum T3. Data are mean ±1 SEM, n=6 rats/treatment group. P=<0.01 Control vs Café.
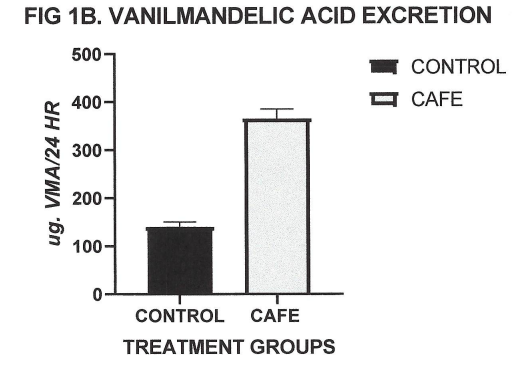
Figure 1B Effect of Café Diet on 24-hour urinary vanilmandelic acid (VMA) excretion. Data are mean ±1 SEM, n- 6 rats/ group. P=< 0.01, control vs café diet.
Measures of resting oxygen consumption were determined in normally fed and cafeteria fed rats after an 8 hour fast and is depicted in Figure 2A and Figure 2B. As depicted in Fig 2A the café regimen resulted in a significant increase in resting VO2 compared to normally fed control or sham rats. Administration of the α-MPT resulted in decreased VO2 in the Café treated animals but was without significant thermic effect in control or sham groups. Because of the potential for the SNS ablation to result in lower body temperatures following α-MPT treatment, a correction based on the Kaplan and Leville24 computational treatment method for differences in body temperature was applied to normalize the thermogenesis data and indicated that the α-MPT induced decreases in colonic temperatures in both the control and café groups, with the greatest decrease in the café group. The α-MPT resulted in an approximate 50% decrease in VO2 in the café group, consistent with the decrease in sympathetic activity following treatment. The remaining difference is presumed to reflect metabolic actions of thyroidal and other biochemical contributors to energy homeostasis (Table 2).
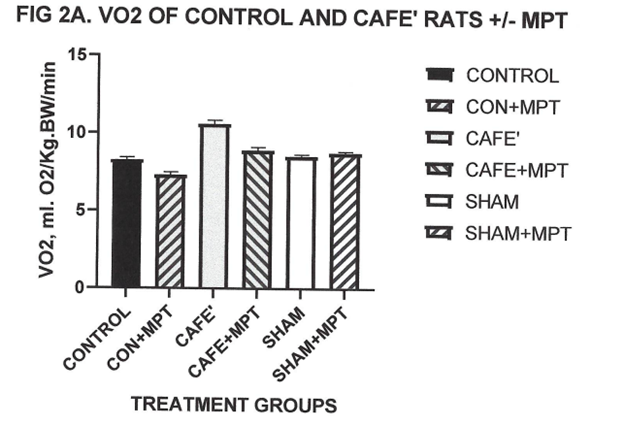
Figure 2A Effect of café diet on resting oxygen consumption at thermal neutrality. Data are mean ±1 SEM, n- 6 rats/ group. P=< 0.05, control vs café diet and control+MPT vs Café + MPT . Sham vs Sham+MPT = N.S.
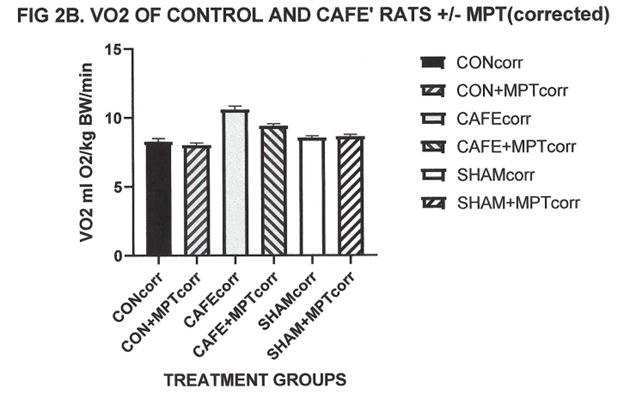
Figure 2B Effect of café diet on resting oxygen consumption at thermal neutrality, corrected for differences in body temperature. Data are mean ±1 SEM, n- 6 rats/ group. P=< 0.05, control vs café diet and control+MPT vs Café + MPT . Sham vs Sham+MPT = N.S.
Temperature, °C |
||||
Group |
N |
Before α-MPT* |
After α-MPT |
Mean % α-MPT Temp Change |
Control |
5 |
37.90±0.20a |
36.51±0.20b |
-1.39±0.34c |
Café |
5 |
38.10±0.26a |
37.50±0.03a, b |
-0.52±0.23b |
Sham** |
5 |
37.50±0.08a |
37.63±0.15a |
+0.13±0.08a |
ANOVA |
|
NS |
NS |
<0.05 |
Table 2 Effects of α-methylparatyrosine on body temperature change of rats
Data are mean + 1 SEM, n= 5 rats/treatment group. [*] = α-MPT or sham injection. Values with a different letter represent Student-Neuman Keuls subgroups at p = < 0.05. All measurements taken after an 8 hour fast.
Temperature values represent colonic temperatures taken with an YSI quick response thermistor apparatus
The effects of the cage diet on circulating T3 are depicted in Figure 1A and show that the café diet resulted in a highly significant increase in circulating T3 concentrations. The effects of the Café diet on 24-hour vanilmandelic acid (VMA) excretion, reflective of net catecholamine metabolites, is depicted n Figure 1B, and show that the Café diet was associated with a marked increase in catecholamine metabolites, reflective of a significant increase in sympathetic activity. Not shown, the α-MPT treatment was without effect on circulating T3 concentrations or VMA excretion, where VMA results were virtually nil.
Measures of resting oxygen consumption at thermal neutrality (30°C) are depicted in Figure 2A and indicate that the café diet resulted in a significant increase in resting VO2 compared to normally fed control or sham treated rats. When the sympathoplegic drug α-methylparatyrosine (α-MPT) was administered however, the magnitude of the increased thermogenesis due to the café diet was only partially decreased, representing approximately only one half of the adaptive increase in VO2. Because body temperature may become decreased following SNS ablation, with resulting decreases in resting VO2, a thermal correction factor as outlined by Kaplan and Leville24 was applied to compensate for the decreased body temperatures. The decreases in body temperature were likely minimal while in the VO2 cannister, but still reflected a near 50 percent decrease in the magnitude of the diet induced increase in VO2 in the café fed rats. The effect of α-MPT on resting VO2 or body temperature changes were nil in the sham group as predicted.
The mechanisms of adaptive thermogenesis in response to adjustments in diet and environment in man and animals are complex and multifactorial, and likely implicate both the sympathetic nervous system in addition to the thyroidal axis and other contributing factors that are contributory to mechanisms of energy homeostasis. In the present study, both sympathetic and thyroidal actions were documented in adult lean LA/Ntul//-cp rats following a prolonged duration of overfeeding via the café diet model approach. Numerous studies have demonstrated an increase in both resting and norepinephrine stimulated thermogenesis in rodent strains following café overfeeding studies, but the partition between the sympathetically mediated vs non-sympathetically mediated contributions remain unclear. Early overfeeding and dietary manipulation have been shown to increase both brown adipose tissue (BAT) mass and cellularity, a prominent tissue in the thermogenic responses to diet and environment, and which contributes to the thermic responses observed in the present study. The BAT tissues have an abundant sympathetic innervation and a robust blood supply, in addition to an abundancy of specialized mitochondria capable of generating heat energy and dissipating it to peripheral tissues where it may be needed to maintain thermal homeostasis and contribute to energy balance. While net caloric intake remains a prime factor is energy metabolism, the macronutrient distribution also contributes an equally vital role in balancing metabolic pathways to conserve energy during undernutrition or to dispel nutritionally induced extremes in energy intake via thermogenesis during perioof ds of excess ingestion or macronutrient imbalance.
This study confirms that long term cafeteria feeding as an overfeeding regimen result in increases in body weight, adaptive thermogenesis, circulating T3 concentrations and 24-hour urinary vanilmandelic acid excretion as a measure of sympathetic activity in a congenic strain of normally lean rats compared to their normally fed and housed lean littermates. In addition, pharmacologic ablation of sympathetic activity via the drug α-methyltyrosine resulted in significant decreases in metabolic rate and in body temperature regulation in both normally fed and cafeteria fed rats. In addition, when the body temperatures of the α-MPT treated rats were factored into the findings with a thermal correction factor, significant decreases in resting metabolic rates equivalent to about 50% pf the increase in thermogenesis remained present, thereby suggesting that the sympathetic blockade could only account for about half of the diet induced increase in thermogenesis. Thus, although the results are qualitatively consistent with a sympathetically mediated increase in diet induced thermogenesis similar to that reported in other studies, the failure of α-MPT to more completely block the diet induced increase in RMR in the Café rats suggests that additional contributors to the adaptive thermic responses are apparent. Thyroidally mediated factors likely represent a significant proportion of the chronic increase in thermogenesis observed. Thyroidally mediated mechanisms in intermediary metabolism typically contribute approximately 45% of the basal metabolic rate in humans, and the elevations in circulating T3 observed in this study suggest that the residual non-sympathetically mediated thermic responses in concert with insulin or other hormonal actions may be at least partially secondary to thyroidally mediated processes in energy metabolism. Both T3 and insulin appear to be essential participants in the expression of DIT, where they facilitate glucose uptake in most peripheral tissues including skeletal muscle and white adipose tissue. The longer duration of the present study likely enabled a more complete physiologic adaptation to the overfeeding regimen. Despite the long duration of the café diet in the present study, excess weight gain was minimal when compared to the same feeding regimen to genetically obese rats, where the rates of weight gain greatly exceeded that of similarly fed lean animals, suggesting that the thermic responses of the Café fed rats likely contributed to the dissipation of a portion of the excess calories ingested.
Thyroxine-5’-deiodinase activity is essential for the extrathyroidal conversion of T4 to the metabolically more active T3 in peripheral tissues and has been shown to be associated with insulin actions in diabetes, where insulin resistance is often common in Type II (NIDDM) and insulin deficiency in Type I diabetes (IDDM).25–27
This process is likely to be important in the regulation of cellular energy expenditure and energy homeostasis, since it results in the intracellular generation of T3 from T4 in close proximity to its nuclear receptor domains which modulate its metabolic sites of action. During periods of caloric excess deiodinase enzymes can facilitate the activation of intracellular thyroidal activity while during periods of caloric deprivation thyroidal actions become diminished via formation of reverse T3 (rT3), a metabolically inactive metabolite of T4. Nutritionally induced increases in T3 concentrations have previously been demonstrated in normally lean rats fed protein restricted diets or the café diet regimen for shorter durations, but the effects of long-term café feeding have not previously been reported. In the protein restricted feeding regimens, where the protein calories were replaced with carbohydrate or fat in a rat model of protein calorie malnutrition (PCM), carbohydrate resulted in the greatest and most long-lasting increases in circulating T3 concentrations, consistent with insulin mediated element in the expression of adaptive thermogenesis. Both the present café diet and the PCM regimen represent high carbohydrate diets, apparently capable of inducing metabolic pathways to minimize the adverse impact of the excess calories in an otherwise unbalanced diet. In contrast, dietary lipid, due to its energetically efficient pathways for luminal uptake and deposition in adipose tissues best represents and energy storage fuel of white adipose tissue (WAT), with only indirect effects on thermogenic activity. Bulowiecki et all have demonstrated that insulin resistance impaired the expression of diet induced thermogenesis. Ravisen and Fanforth demonstrated that glucose infusion as a single macronutrient could induce thermogenesis in human subjects, thereby clarifying a role for glucose mediated activation of whole body thermogenic mechanisms and Sims et al demonstrated that glucose administration in fasting subjects could switch the conversion of rT3 to T3 only minutes after the administration of the glucose ingestion. In recent studies, 14 hours of cold exposure also resulted in significant increases in circulating T3 in lean rats, thus the contribution of T3 to thermic responses to diet and environment are well established and likely played a significant role in the present study. The results imply that both sympathetic and thyroidal mediated mechanisms contribute essential elements to the thermic responses to diet and environment and may complement each other in a somewhat reciprocal manner.
Thus, the present study demonstrates the presence of multiple metabolic components in the development and expression of DIT in a normally lean animal. Although not measured in the present study, the likely contribution of BAT to the phenomena of DIT may be envisioned to represent an acute response to challenges by diet and environment and to facilitate the expenditure of excess or unneeded calories as heat to enhance survival of the animal during extended duration of overnutrition or during the thermic demands of cold exposure. The specific roles of the increases in circulating T3 concentrations during overfeeding or nutritionally unbalanced dietary regimens are only speculative but may also include an enhancement of BAT development and activity in addition to increasing other avenues of energy generation and expenditure. Increases in non-sympathetically mediated aspects of energy metabolism are likely to occur more slowly than sympathetically mediated mechanisms but owing to their nuclear receptor-activated sequence of events likely have a more gradual onset, to include liver and other tissues where oxidative metabolism occurs, and thus to impart a more long-lasting impact of resting metabolic rates and energy homeostasis. Regardless of the mechanism involved, the non-sympathetically mediated elements of energy expenditure appear to contribute a considerable proportion of the adaptive responses to the café diet, while in normally fed rats the sympathetic contribution was modest.
The author thanks Seth Wolpert and Robin Bye for assistance in data collection and analysis during their research elective, David Tulp for assistance with the animal husbandry, and the late Otho Michaelis IV for constructive comment on this experimental protocol. Supported by Grants in aid from Colby College, Drexel University, and Institutional resources of USAT Montserrat.
None.
The authors declare no conflict of interest.

©2022 Tulp. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.