eISSN: 2473-0815


Research Article Volume 10 Issue 1
1Department of life sciences, Faculty of Sciences of Bizerte, Tunisia
2Department of life sciences, Faculty of Sciences of Gafsa, Tunisia
3Laboratory of Pharmacology, Medicine Faculty of Sfax, Tunisia
Correspondence: Ben Nasr Hmed, Department of Life Sciences, Faculty of Sciences of Gafsa, Tunisia
Received: July 05, 2022 | Published: September 8, 2022
Citation: Jamal G, Hafsia B, Abir J, et al. Diabetes ethnopharmacology in rural region: study of a case report and review of literature. Endocrinol Metab Int J. 2022;10(1):41-45 DOI: 10.15406/emij.2022.10.00317
Recently, there is an ascendant recourse for medicinal plants’ utilization as treatment of several chronic diseases, including diabetes. While effective in wound healing, some herbs might present toxic effects at certain doses or when used for long periods. In this study, the frequently used antidiabetic herbs, in a rural region, was reviewed with special focus on their possible toxicological features
Keywords: herbal medicine, toxicological features, diabetes.
Diabetes mellitus (DM) is a worldwide chronic disease. Recent data shows that its prevalence reached about 463 million cases, in 2019. The incidence of DM presents important regional disparities, and its etiology includes multiple risk factors such as nutritional habits, age, gender, obesity, physical activity, behavior, and many other factors.1 DM is mainly characterized by chronic impairment of glucose turnover as a consequence of cellular insensitivity to insulin or the lower levels of its secretion by pancreatic β-cells. DM types and subtypes are defined according to its etiology and clinical findings.2–4 The disease is usually associated to other pathologies such as Alzheimer,5 retinopathy,6 cardiovascular diseases,7 obesity,8 and nephropathy.9 DM management involves both pharmacological and non-pharmacological interventions, in order to reduce the blood levels of glucose through stimulating its cellular uptake and regulating its metabolism.10
Phytotherapy is the oldest medical practice throughout the Human history, among which Chinese medicine and Indian ayurveda are the best known. Recently it gains great scientific and clinical interest because of its medical virtue, disponibility for all populations and lower cost. In particular, the anti-diabetic herbarium comprises hundreds of plant species with approved wound healing effects.11,12 Nonetheless, some plants species might present risk of intoxication that should be considered in order to ensure patients’ safety. The variability in plants’ distribution and disponibility, and in populations’ culture might influence the choice of the used plants and techniques of their preparation as therapeutics. In such view of point, this work discusses the antidiabetic herbarium of a rural region from Tunisia.
Determination of the anti-diabetic herbarium
In order to determine the used medicinal plants to treat diabetes, a questionnaire was administered to diabetic patients belonging to a rural South-Western village (Madjel Belabbes) of Tunisia, from Marsh to April 2021. This region is settled near the Chaanbi mountain which is characterized by high plant’s diversity. Among 91 diabetic patients registered at the hospital of the village, 26 DM type 1 and 62 DM type 2 patients were enrolled in this prospective study (two childs and one pregnant woman were excluded from the study). All included participants gave a signed informed consent. Patients’ sociodemographic parameters, disease’s description and clinical findings are summarized in Table 1 and 2. Participants were asked, by direct interview, about their usage of medicinal plants to treat diabetes (what plant species do you utilize to treat diabetes? How do you prepare the plant for treatment? How do you apply (intake) this plant preparation? How many times do you intake the plant preparation per week? Did you manifest discomfort or signs of intoxication (e.g., vomiting, headache, nausea, fever, etc.) following the intake of the plant’s preparation? If yes, what are these signs? Did you have a physical visit because of the developed signs of intoxication?). All related responses did concern the last two months anterior to the questionnaire date. A complete description of the disease’s status (type of diabetes, age at diagnosis, associated complications, etc.) was also realized. The hematological and biochemical profiles of patients were retrospectively analyzed using the most recent medical report effectuated within the last two months. Diastolic (PD), systolic (PS) and mean (PM) arterial blood pressures were also registered, for each patient. Chi- square test was used to define significant associations between the studied parameters. To compare mean values of the measured parameters between, one-way ANOVA test followed by LSD test was effectuated. SPSS for Window.17 (IBM corporation) has been used to carry out statistics with fixed confidence interval of 0.95.
Phytochemical characterization of anti-diabetic plants
The content in phenols, flavonoids and tanins were determined for dried fenugreek seeds and leaves of Thynus vularis, Rosmarinus officinalis, Artemisia herba alba and Melissa officinalis following methods described by Bouzenna and golleagues (2021),13 Aqueous extracts were obtained by 72 hours’ water- maceration of the finely grinded parts of plants. Folin- Ciocalteu’s reagent was used for total phenolic content measurement. Aluminium chloride (AlCl3) and Vanillin ware respectively utilized to measure flavonoids and condensed tanins contents. Results are expressed as the absorbance of the respective solutions using UV- visible spectrophotometer, respectively at 725 nm, 510 nm and 500 nm, respectively for phenols, flavonoids and condensed tannins.
Data review
In agreement to the questionnaire’s results, a search across scientific databases was carried out in order to outline the toxicological patterns of the frequently used herbs to treat DM. Diabetes, medicinal plant, herb, phytotherapy, intoxication, toxicity, pharmacology, clinical findings, and the plant scientific names, were the used key words in this search.
The majority of participants in this observational study were undergraduated (88.6 % have primary school levels) and of low income. 52 patients presented familial history of diabetes and 42 ones have associated health’s complications (hypertension, obesity, dyslipidemia, anemia, and cardiopathy). 7 patients presented diabetes with two or more associated complications. In exception of three DM type 2 cases, all subjects regularly use their conventional pharmaceutics (Table 1).
Age (years) |
53.51±16.88 |
|
Sex ratio (H : F) |
0.96 |
|
BMI (Kg.m-2) |
25.81±3.87 |
|
Overweight |
43.13 % (38) |
|
Obesity |
12.50 % (10) |
|
School level |
≤ 6 years |
88.60 % (78) |
>6 years |
11.36 % (10) |
|
Incomes |
low |
93.20 % (82) |
Medium |
06.80 % (6) |
|
Smoker |
10.20 % (9) |
|
Type of diabetes |
DM1 |
29.50 % (26) |
DM 2 |
70.50 % (62) |
|
Diabetes’ duration (years) |
8.67±8.64 |
|
Familial history of diabetes |
59.10 % (52) |
|
Complications |
None |
52.27 % (46) |
Hypertension |
26.14 (23) |
|
Dyslipidemia |
14.77 (12) |
|
Cardiopathy |
05.68 % (5) |
|
Coagulopathy |
01.14 % (1) |
|
Anemia |
01.14 % (1) |
|
Mutiple complications |
07.95 % (7) |
|
Drugs’ disponibility |
Yes |
96.59 % (85) |
Non pharmacological treatment |
Yes |
56.82 % (50) |
Phytotherapy |
Yes |
55.68 % (49) |
Regular (> 3 times / week) |
20.41 % (10) |
|
Irregular (≤ 3 times /week) |
79.59 % (39) |
Table 1 Sociodemographic and physical description of diabetic patients
A total of twenty-nine plant species were utilized, in different forms, alone or in combination, to treat diabetes and its complications. They belong to different families : Fabaceae (Trigonella foenum graecum, Cicer arietimum, and Retama reatam), Lamiaceae (Rosmarinus officinalis, Thymus vulgaris, Melissa officinalis, Mentha sp, Ajuga chamaepitys and Marrubium vulgare ) Rosaceae (Malus pumula, Prunus dulcis) Rutaceae (Citrus limon, Citrus xsinensis), Asteraceae (Artemisia herba alba), Ephedraceae (Ephedra alata alenda), Aparagaceae (Hyacinthus orientalis), Lauraceae (Cinnamomum verum), Apiaceae (Foeniculum vulgare) Saxifragaceae (Saxifraga sp), Amaryllidaceae (Allium sativa), Myrtaceae (Syzygium aromaticum), Oleaceae (Olea europea), Cucurbitaceae (Citrullus colocynthis), Zingeberaceae (Curcuma longa), Cupressaceae (Tetraclinis articulata), Theaceae (Camellia dulci), Schizandraceae (Illium verum), Ranunculaceae (Nigella sativa), Verbenaceae (Vitex agnus castus), Globulariaceae (Globularia alypum) (Figure 1).
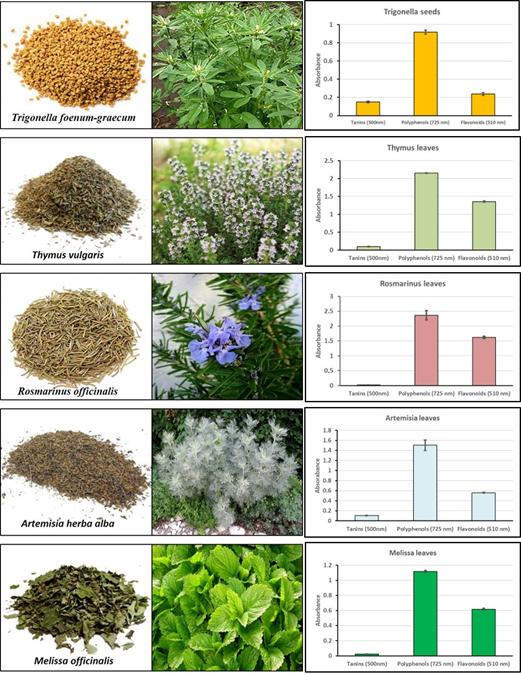
Figure 1 Plant species used as antidiabetics and their total contents in tannins, polyphenols and flavonoids (expressed by their absorbance).
The recourse to phytotherapy was observed in 55.68% of cases, among them 20.41% regularly used medicinal herbs. Trigonella foecum graecum was the most used antidiabetic plant (71.43 %) irrespectively from the DM type and the presence of associated diseases. Cinnamomum verum, Melissa vulgaris, Artemisia herba alba, Rosmarinus vulgaris and Thymus vulgaris form a second line to cure diabetes. Other species were less frequently utilized in a discontinuous manner. T. foecum graecum seeds were either directly consummed or powdered and suspended in drinking water and used as beverage. Thymus, Rossmarinus and Artemisia aerial parts are mainly used as tea- infusion or culinary spice. In rare cases, patients did take the water- macerate of the plant’s leaves
Chi- square test revealed significant association between phytotherapy and diabetes’ complications (ꭓ2 = 4.299, p=0.036). In particular, diabetes mellitus type 2 patients did intake infusions of Melissa, Thymus and Rosmarinus more frequently when they manifest hypertension (ꭓ2=4.299, p=0.038, ꭓ2=8.750, p=0.015 and ꭓ2=8.750, p=0.015; repectively). Fenugreek was utilized independently from DM type and the presence or not of health’s complications. This suggests that Trigonella served only for its antidiabetic properties. Other plants, however, are intended to ameliorate the general health status through preventing or healing associated diseases. The clinical findings revealed significant increase in creatinine content and counts of red and white blood cells in patients auto-medicated by A. herba alba in comparaison to those how did not utilize medicinal plants. Most participants (7 per 10) receiving Melissa officinalis have a disequilibrated DM type 1 (ꭓ2 = 6.521, p = 0.011) as diagnosed by the augmented levels of glycosylated hemoglobin (HbA-c). In exception of a woman who presented vomiting and nausea immediately following intake of fruits’ infusion of Citrullus colocynthis, none of patients did experience intoxication signs or discomfort (Table 2).
Medicinal herb use |
Frequently used plant species |
||||||
(Number of patients) |
No (39) |
Yes (49) |
Trigonella (35) |
Artemisia (12) |
Thymus (13) |
Rosmarinus (13) |
Melissa (10) |
Age (yrs) |
52.1±17.6 |
56.4±14.0 |
55.4±15.6 |
57.8±14.4 |
58.0±7.5 |
56.7±6.0 |
58.7±8.0 |
age at diagnosis (yrs) |
44.9±17.3 |
47.7±15.2 |
47.7±16.6 |
49.3±18.7 |
49.9±16.0 |
48.8±8.6 |
48.8±8.7 |
BMI (Kg.m-2) |
25.7±3.5 |
26.1±4.1 |
25.5±4.3 |
27.7±3.3 |
26.9±4.6 |
26.3±4.4 |
26.3±3.9 |
hematology |
|||||||
RBC (106.mL-1) |
4.92±0.46 |
5.14±0.52 |
5.06±0.53 |
5.41±0.63 |
5.28±0.78 |
5.06±0.55 |
5.15±0.23 |
WBC (103.mL-1) |
8.44±1.77 |
9.23±4.99 |
9.52±5.62 |
11.60±5.79 * |
8.81±1.56 |
7.84±1.50 |
6.90±2.37 |
PLT (mL-1) |
272±67 |
260±92 |
251±92 |
303±96 |
244±80 |
229±72 |
244±63 |
HGB (g.dL-1) |
12.87±1.55 |
12.99±1.53 |
12.58±1.51 |
13.35±1.33 |
13.40±1.56 |
13.09±1.44 |
12.98±0.74 |
HbAc % |
7.13±2.41 |
8.41±1.48 |
8.45±1.76 |
8.55±1.77 |
8.60±1.92 |
8.40±1.25 |
8.09±1.32 |
biochemistry |
|||||||
GLUCOSE (mmol.L-1) |
11.65±4.98 |
12.29±4.59 |
12.75±4.62 |
13.39±5.05 |
13.18±4.83 |
13.09±12.04 |
|
CHOLESTEROL (g.dL-1) |
4.52±0.83 |
4.81±1.02 |
4.67±1.09 |
4.81±1.03 |
5.10±1.17 |
5.18±1.06 |
5.09±0.48 |
CREAT (mol.L-1) |
92.48±26.72 |
99.55±28.85 |
102.5±29.45 |
149.10±41.16 * |
91.0±28.70 |
83.54±28.76 |
73.17±8.08 |
TRIGLYCERIDS (g.dL-1) |
1.64±0.65 |
2.27±1.43 * |
2.27±0.97 |
2.50±1.32 |
1.86±1.51 |
2.06±1.64 |
2.58±1.85 |
UREA (mol.L-1) |
5.45±2.88 |
5.72±4.80 |
6.18±2.74 |
7.46±1.89 |
5.71±3.20 |
5.61±3.10 |
4.12±0.71 |
AST (IU) |
12.13±3.52 |
20.63±8.07 |
19.71±8.26 |
15.50±6.36 |
24.75±10.01 |
23.0±9.51 |
19.6±9.06 |
ALT (IU) |
17.25±5.31 |
21.67±7.71 |
20.14±8.19 |
18.0±7.94 |
25.25±9.91 |
23.60±9.34 |
19.59±6.88 |
hemodynamic |
|||||||
PS (mmHg) |
12.28±1.21 |
12.31±1.06 |
12.17±1.04 |
12.08±3.35 |
12.50±1.09 |
12.31±1.25 |
12.40±0.97 |
PD (mmHg) |
7.06±1.09 |
7.06±0.94 |
7.0±0.97 |
7.00±1.04 |
7.25±1.06 |
7.0±1.15 |
6.90±0.88 |
PM (mmHg) |
8.80±1.11 |
8.81±0.95 |
8.79±1.01 |
8.70±1.09 |
9.00±1.04 |
8.77±1.17 |
8.74±0.87 |
FC (bit.min-1) |
70.67±2.83 |
69.73±2.93 |
70.19±2.97 |
69.0±2.96 |
69.8±2.98 |
69.54±3.04 |
69.40±2.07 |
Table 2 Hematological, biochemical and hemodynamic parameters of diabetic patients using or not herbal medicine
(*): significant differences in comparaison to patients who did not use herbs (no herbs).
According to Asadi and colleagues (2019), the intake of Melissa officinalis improves the control of diabetes by metformin and leads to HbAc reduction.23 Since most patients using M. officinalis are treated by insuline, it is suggested that this plant synergistically acts with metformin but not insuline, fact that explains our observed results. Several studies proved the beneficial effect of medicinal plants, including Trigonella foenum graecum,23 Artemisia herba alba,18,21 Rosmarinus offiicinalis,25–27 Thymus,29,30 and Melissa31 in ameliorating the diabetic status. Specifically, great consensus is brought back for the antidiabetic activity of fenugreek that was reviewed elsewhere in EMIJ.32 Their richness in various chemicals permits different mechanisms of action to lower blood sugar’s concentration such as, stimulating insulin secretion, inhibiting amylase, glucosidase and phospho-fructokinases, and facilitating glucose uptake by cells.27–35 Substances contained in medicinal herbs’, essentially polyphenols and flavonoids, are also known to repress oxidative stress that mediates cellular damages and forms a cross- link mechanism for developing associated health disorders.25 Obviously, most of these herbs are considered as generally safe and can improve many types of metabolic disorders. They can be used as alternative or adjunct for conventional therapy.
Clinical and experimental studies approved the synergism between fenugreek and metformin in reestablishing glycaemia and lipids’ profile in diabetes. This encourages its intake as dietetic supplement to manage the disease and its associated.36–38
In raison of the frequent use of medicinal herbs and unawareness of the general population about their effectiveness, safe doses and toxicological features, some sporadic intoxications have been observed. Ouzir and colleagues (2016) reviewed these properties for fenugreek and found that its LD50 in experimental animals exceeds 3.5 g.kg-1 of body weight. This toxicity considerably varies depending on the extraction’s procedure. Clinically, there was only transient nausea, stomach discomfort and diarrhea that were noticed following long-terme intake of 25 g per day of fenugreek’s seeds. Chronic exposure of mice or rats to higher doses of Trigonella extracts resulted in multi-systemic disturbances including convulsion and loss of implantation.39 Similarly, bare intoxication symptoms have been observed using Rosmarinus officinalis,40,41 Thymus vulgaris,42 Artemisia herba alba43 and Melissa officinalis,44 in animals exposed to very high doses. Other medicinal plants found in this census such as Cinnamon,45,46 Globularia47 and Citrullus colocynthis,48,49 showed great potential to counteract diabetes. Obviously, they did present toxicity related to the reproductive system and fertility, when administered at high doses to experimental animals.50,52
In this observational study we found 29 plant species that are used to treat diabetes mellitus and its associated pathologies, like hypertension. Such medical virtue is scientifically approved. However, efficacious and safe quantities that patient should intake are still unknown. Experimental models revealed reproductive and fertility related intoxication symptoms at very high doses.39,50–52 Some allergic reactions to specific vegetal products did also manifest following intake of these herbs. According to experimental findings reported in the literature, it is suggested to avoid auto-medication using herbal medicine for allergic patients and pregnant women. Further prospective toxicological studies are envisaged to better clarify real pharmacological and toxicological patterns of these phyto-therapeutics.
None.
The author declares there is no conflict of interest.

©2022 Jamal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.