eISSN: 2473-0815


Case Report Volume 10 Issue 1
Ministry of Higher Education, Iraq
Correspondence: Saadi JS AlJadir, PhD, MRCP, FACE, FRCP (London), FRCP (Glasgow), Ministry of Higher Education, AlMansour, AlMutanabi, District 607, Alley 20, House 9, Baghdad, Iraq
Received: March 08, 2022 | Published: April 4, 2022
Citation: AlJadir SJS. Case report and disease review: Tophaceous gouty arthropathy. Endocrinol Metab Int J. 2022;10(1):4-12 DOI: 10.15406/emij.2022.10.00312
Gout is a common inflammatory and metabolic disorder of the joints and probably other organs, especially the kidneys. It has a definitive genetic and environmental background, making it mainly a disease of middle-aged and elderly males, infrequently inflicting postmenopausal and elderly women who usually have arterial hypertension, renal impairment, and usually on diuretics.
Excessive tissue urate turnover and persistent hyperuricemia is the hallmark of the disease. A typical algorithm is characterized by acute attack of the monoarticular joint, the metatarsophalangeal joint of the big toe often is involved (podagra), but tarsal joints, ankles, and knees might also be affected.
Chronic asymmetric polyarticular arthritis that might be confused with classical Rheumatoid Arthritis might be encountered in some patients and in recurrent and relapsing diseases. In this setting, many organs and tissues are affected by the deposition of monosodium urate (MSU) crystals other than synovium, bursae, tendons, and periarticular tissues. The risk of involvement of renal interstitium or uric acid nephrolithiasis has a particular interest in the course of the disease.
By the inflammation and collection of MSU crystals in form of tophi that might involve many tissues and occasionally the pinna of the ears, this kind of tophaceous gout is rarely observed nowadays, especially in our community (Middle East region). The patient who is presented here has exhibited acute attack on the top of chronic tophaceous gouty arthritis.
The recent epidemiologic reports revealed that gout has given different results. This wide variation is attributed to the population studied and methods employed, but overall, for the prevalence of <1% to 6.8% and an incidence of 0.58-2.89 per 1,000 person per year. The most noticeable risks for gout are obesity and associated metabolic syndrome (insulin resistance, hypertension, dyslipidaemia), dietary factors, high fructose-containing diet, high purine diet (red meat, internal organ’s meat ,seafood) high consumption of alcohol, and exclusively beer ( as in our patient’s case ), a wide variety of disorders that are characterized by high urate turnover like myeloproliferative disorders, neoplasms, psoriasis, haemolytic anaemias, medications , to under-secretion of urate like renal insufficiency.
In the Ebbers and Edwin Smith Papyri (ca. 1552 bc), in ancient Egypt gout was described. 1000 years after the Ebbers and Edwin Smith Papyri were written, Hippocrates (ca. 460 bc to 370 bc), who had studied the disease and differentiated it from rheumatism and given the term “podagra”. The Roman gladiatorial surgeon Galen described gout as a discharge of the four humors of the body in unbalanced amounts into the joints. Writing ca. 30 AD, Aulus Cornelius Celsius appeared to recognize many of the features of gout, including its link with a urinary solute, late onset in women, linkage with alcohol, and perhaps even prevention by dairy products.
In modern times, A. Leeuwenhoek described the needle-shaped urate crystals under the microscope in 1679. Later in the 1800s, A. Garrod discovered that the presence of excess uric acid in blood is the main culprit for gout.
A 55-year-old Male, (South Pacific, descendant) had been admitted on December 17th, 2006, to medical unit, for being unable to walk for the last 2 days. He had found difficulty in moving his right arm with pain and swelling of the left and right foot, right hand, and elbow. Pain and swelling were associated with fever and malaise.
He had not reported trauma but had a history of road traffic accidents 3 years ago, surgical reduction for tibial fractures was done and transfused 2 units of blood, afterward, he had good healing and recovery. He was a widow with one daughter, 19 years old. He is a usual alcohol drinker, frequently beer, with occasional social binges. He was not a smoker or consuming any other drugs.
He had polyarthritis for the last 10 years, no urinary complaints, he was hypertensive on verapamil 120 mg /day, no other medications. He described his joint problem as painful episodes that come on monthly intervals but are quite milder than the current attack and usually triggered by alcohol indulgence. He had no family members of same condition and no history of diabetes or rheumatologic disorders in the family. He is a well-educated electrician with good income.
Clinical impression
Musculoskeletal examination
Hands (Figure 1)
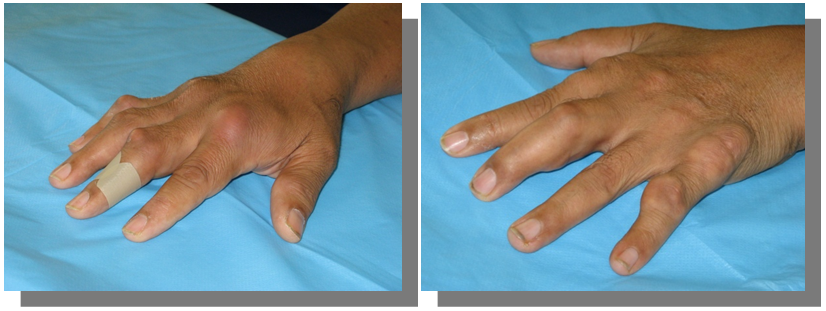
Figure 1 Inflamed dorsum of the right hand, skin redness, swelling, and tenderness. Multiple swellings on left hand.
Elbow (Figure 2)
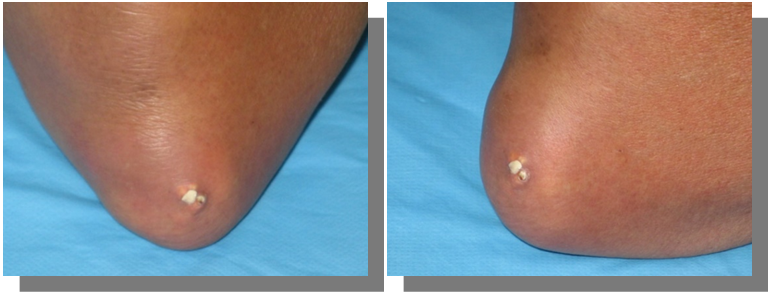
Figure 2 Left elbow had swelling, redness, tenderness, and restriction of movements. Right Elbow had big tophi, soft, rubbery with overlying chalky cheesy material.
Foot (Figure 3)
FBG 8.2 mmol (147ml/dl), HbA1c 6.8, Hb 14,8 g/dl, WBC 19.8 x10/L (Neutrophils 84%, platelets 321x10/L), Serum Electrolytes Na 147mmol /L, K 3.8, HCO3 27, BUN 8.1mmol/L
Liver function tests were normal, except γGT was 32 (upper limit of normal). Serum Triglyceride 3.6mmol (320mg/dl), total Cholesterol 6.24 mmol, LDL 3.6, HDL 1.61.S. uric acid 614 µmol/L (N 140–340), CRP 384mg/L and ESR 93mm/hour were high
Abdomen U/S was normal, Kidneys were normal size, no calcifications, or calculi.
Plain radiographs (Figure 4) (Figure 5)
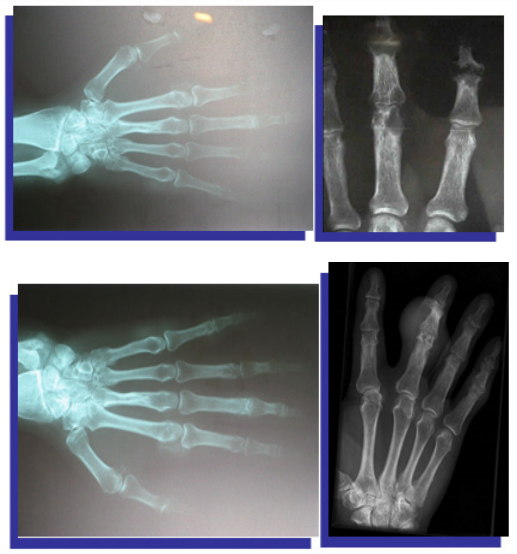
Figure 4 X-ray of both hands shows eccentric juxta-articular soft tissue nodule associated with adjacent bony erosion (rat-bite erosion) with sclerotic margins and overhanging edges invoking the proximal interphalangeal joint of middle finger with normal bone mineralization.
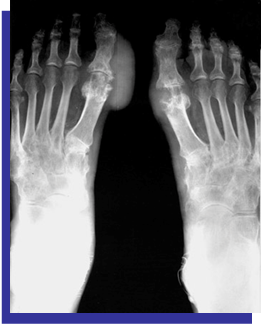
Figure 5 X-ray of both hands shows eccentric juxta-articular soft tissue nodule associated with adjacent bony erosion (rat-bite erosion) with sclerotic margins and overhanging edges invoking the proximal interphalangeal joint of middle finger with normal bone mineralization.
Clinical diagnosis
Acute flare of chronic tophaceous gout
Diagnosis is not based on the level of Serum uric acid, as it should be based on aspiration of synovial fluid and looking under polarized microscopy.
[demonstrate crystal of MSU & identified by strong negative birefringence]
Management
Disease review
Gout is a common inflammatory and metabolic disorder of adult population, that affects the articular and periarticular tissues, it is usually manifested by a wide variety of clinical features in the setting of basic biochemical abnormality of excess tissues saturation of uric acid or persistent hyperuricemia.
The mainstay of biochemical changes that heralds to acute and abrupt peripheral joint involvement that majority of patients are presented, which is attributed to tissue deposition of MSU crystals in joints and probably other tissues, this deposition with eruption of inflammation is the hallmark of the disease; gout
Gout differs from most chronic arthroses in being a treatable disease, especially if :
Pathophysiology
Gout is caused by supersaturation of the target tissues by urate crystals, usually associated with persistent serum hyperuricemia for a variable period.
The mainstay of treatment is serum urates lowering numerically maintaining serum uric acid less than 6mg /dL, this crucial measure can effectively prevent flares and ultimately prevent a recurrence.
However, many individuals might have hyperuricemia and also have urate crystals in their synovial fluids, yet they don’t have gout (by definition clinically) or joint inflammation.
Therefore, the presentence of crystals in the joints and adjacent tissue is not the hallmark of the disease and is not whole disease pathology. For decades there was extensive debate about the inflammatory eruption after the deposition of urate crystal in the target tissues, time is variable for a reason that is not precisely elucidated.
Pro-inflammatory cytokines have a critical role in initiating the cascades of inflammatory reactions to the presence of MSU crystals.
Recent attention has focused particularly on the role of IL-1. This aspect has opened the door for new therapeutic perspectives.
Inciting inflammation in the target tissues is hypothesized by a coating of the crystals with IgG fragments which are pro-inflammatory, as inflammation subsides apoprotein B particle (or might be Apo E ) displaces IgG from the crystals surface.
Renal involvement of gout is of particular interest during this disorder and might include:
Etiology
Hyperuricemia occurs when there is an imbalance between excess uric acid production, and stores (overproduction ) in the setting of lag behind renal excretion ( underexcretion ), persistently elevated urate in serum will be crystallized under certain conditions ( temperature, PH ..etc.) and ultimately deposit in target tissues.
Over-production disorders
These disorders include
Risk factors for the development of gout
There are several acquired and environmental factors that may increase the risk of developing hyperuricemia and gout:
Genetics
Clinical features
Acute arthritis
The initial and classical manifestation of gout is usually an acute attack in the majority of 90 % of patients, usually single or occasionally 2 joints and more might be affected and characterized by abrupt onset of severe pain and swelling, in more than 50% lower extremities small joints. Clinical scenario of the acute attack takes the crescendo pattern in 4-12 hours. The classical acute gout of 1st metatarsal phalangeal joint (Podagra) . the affected joint is erythematous and swollen with exquisitely tender, less frequently ankle joint might be affected. Podagra is not pathognomonic to acute gout another form of arthroses might take the same presentation. The joint inflammation usually takes 7-10 days and then subsides. In 10% of patients are presented initially by polyarticular arthritis that might mimic chronic arthroses, and occasionally creates difficulty in reaching the precise diagnosis. The attack might recur after a very variable period during this interval the joint looks normal, intermittent periods may extend months or even years, and few reported cases on flare at all! (Figure 6).
Chronic gout
If the condition is left untreated, hyperuricemia persists, most patients develop more frequent acute attacks, less intense usually but may affect multiple joints at the same time or in rapid succession. Over time there will be subsequent deposition of MSU crystals in the joints, tendon sheaths, over bony prominences, and in subcutaneous tissues called tophi. Tophi are recognized as hard swellings whitish to yellow coloured under the skin, some may break and discharge chalky material containing MSU crystals. they have a predilection to common sites; the ear pinnae, olecranon or prepatellar bursae, the distal interphalangeal joints, the dorsum of the MTPJ, and metacarpophalangeal joint (MCPJ) (Figures 7–9).
Renal manifestations
Gout could be considered a primary renal disease, as the renal under-secretion of excess urate is considered the essential defect in hyperuricemia. The consequences of gout and hyperuricemia are also manifest in the kidney and comprise several clinical syndromes These may be considered in the order in which they appeared historically: uric acid nephrolithiasis, urate nephropathy in the absence of overt lithiasis, acute uric acid-related nephropathy due to tumor lysis with use of cytotoxic chemotherapy, and, most recently, the notion; soluble urate may have a direct toxic effect on renal parenchyma and vasculature this may evolve into urate nephropathy.
Uric acid nephrolithiasis
Uric acid was first identified as a component of kidney stones and it is comprised of 10 % of nephrolithiasis in the United States. Uric acid stones are the main reason for nephrolithiasis in individuals with type 2 diabetes mellitus and obesity.
As a matter of interest, uric acid stone formers tend to be older and more likely to be obese than calcium oxalate stone formers.
Comorbidities and metabolic syndrome
In the majority (more than 75% of cases) gout exists in the association of multiple comorbidities, many of which comprise metabolic syndrome; hypertension, dyslipidaemia, insulin resistance, Inflammation, high waist circumference (abdominal adiposity), and atherosclerotic vascular disease (Figure 10).
Uric acid is synthesized in the liver, intestines, and endothelium from purines. Fructose which had been consumed increasingly in past decades increases intracellular urate production as well as a culprit to insulin resistance.
Serum urate levels also depend on the degree of excretion by the kidneys, and low excretion is the main factor contributing to high urate levels.
The most recent epidemiologic studies have shown robust evidence towards a higher prevalence of metabolic syndrome and its components (especially high triglycerides and waist circumference..) in individuals with hyperuricemia and gout compared with controls.
Differentials
Acute monoarthritic
Polyarticular arthritic
Rheumatoid Arthritis, Psoriatic, Reactive arthritis, Sarcoid …
Laboratory studies
Laboratory testing: The detection of MSU crystals in synovial fluid using polarizing microscopy is diagnostic of gout and the preferred approach for diagnosis. MSU crystals are needle-shaped and negatively birefringent. The absence of these crystals does not necessarily eliminate the possibility of gout, but it makes gout less likely (as reflected by a negative value). Although this method is preferred for diagnosis, it is not always feasible, extreme tenderness for touch and technically joint aspiration may be difficult in small joints, moreover polarized microscopy might be not available in all laboratories!
As such, MSU crystal detection is an unfeasible universal diagnostic standard, but this method of evaluation should be used when possible.
Serum uric acid
In patients with gout, serum urate concentrations are generally elevated; however, serum urate levels are not always elevated at the time of an acute gout flare.
Ideally, serum urate is measured >4 weeks after an acute attack provided treatment with urate-lowering therapy has not commenced yet.
Synovial fluid examination
A sample of fluid for gram stain and culture, to differentiate from septic arthritis
Blood tests for White Cell Count and CRP, other tests like ANF, Rheumatoid Factor, and tests for immune arthritis might be required.
Imaging studies
Joints
Bones
Surrounding soft tissues
Ultrasound study in gout
In recent years the US had gained interest due to improved techniques, no radiation exposure, and cheap cost.
Tophi tend to be hyperechoic, heterogeneous, and have poorly defined contours. other findings may include:
CT scan
Findings generally reflect those on the plain radiograph
Dual-energy CT can distinguish between urate mineralization and calcification, especially critical in atypical cases.
It can quantify MSU load, therefore, can monitor treatment.
MRI has been increasingly utilized in some centres.
Signal characteristics of gouty tophi are usually:
Treatment
NSAIDs
Prophylaxis to prevent acute flares
Colchicine
Allopurinol
Approximately 3-10% of patients taking allopurinol develop dyspepsia, headache, diarrhoea, or pruritic maculopapular rash, infrequently, patients can develop allopurinol hypersensitivity, which has a mortality rate of 20-30%...Prior to initiation of allopurinol, rapid polymerase chain reaction-based HLA–B*5801 screening should be considered as a risk management component some genotypes both the HLA–B*5801 are susceptible to a severe allopurinol hypersensitivity reaction
Lowering uric acid levels:
Dietary Advice
Dietary & lifestyle recommendations
Recommendations |
Benefits |
|
|
|
|
DHA, octadecanoic acid; EPA, eicosatetraenoic acid; MSU, mono sodium urate
A comprehensive treatment strategy needs to be initiated.
None.
The authors declare no conflict of interest.

©2022 AlJadir. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.