eISSN: 2473-0815


Research Article Volume 11 Issue 2
Department of Endocrine Surgery and Radiology, Churchill Cancer Centre, Oxford University Hospitals NHS Foundation Trust, UK
Correspondence: Radu Mihai, Consultant Endocrine Surgeon Department of Endocrine Surgery Churchill Cancer Centre Oxford, OX3 7LE, United Kingdom, UK
Received: July 07, 2023 | Published: August 1, 2023
Citation: de Jong MC, Khan S, Boardman P, et al. Adrenal venous sampling fails to identify co-secretion of aldosterone and cortisol in patients with primary hyperaldosteronism. Endocrinol Metab Int J. 2023;11(2):30-35 DOI: 10.15406/emij.2023.11.00328
Purpose: Urine-steroid-profile studies suggest that an up to 10% of aldosterone-producing adrenal adenomas co-secrete cortisol, a phenomenon labelled as Connshing. Although it is exceedingly rare to observe hypoadrenalism after adrenalectomy for Conn’s syndrome, recent papers suggest that formal assessment of cortisol co-secretion should be undertaken preoperatively in all patients with primary hyperaldosteronism. This study aimed to verify if cortisol levels measured during adrenal venous sampling (AVS) could identify patients with Connshing
Methods: Retrospective audit of 92 patients who underwent AVS for lateralization of aldosterone-secreting adenomas.
Results: Between June2014-March2020 a total of 92 patients (60M:32F, age 53±11 years) were diagnosed with Conn’s syndrome, and had lateralization confirmed on AVS, before proceeding with laparoscopic (n=32) or retroperitoneoscopic (n=60) adrenalectomy.
Median values for Selectivity Index were 13.2 on the right side (IQR:8.1-23.2) and 11.1 on the left side (IQR:8.1-16.0), without evidence that right side was less likely to be cannulated. The Lateralisation Index had a median value of 12.9 (IQR 6.6-29.0).
Absolute values for cortisol concentration varied widely both on the dominant side (median:8954 nmol/L; IQR:5535-12207)), and on the non-dominant side (median: 6684nmol/L; IQR:4458-10547). There was no evidence of inhibition of contralateral secretion, as no patient had cortisol levels in the non-dominant side lower than the peripheral concentration.
Conclusion: This study found no evidence that Conn’s patients might have same-side dominant co-secretion of cortisol. Cortisol-levels in samples collected during AVS cannot identify patients who might benefit from a formal preoperative assessment of the hypothalamic-adrenal axis.
Keywords: primary hyperaldosteronism, co-secretion, cortisol, Conn’s adenoma, aldosteronoma
There is increasing interest in identifying patients with primary hyperaldosteronism (PA) from the large cohort of hypertensive patients, as specific treatment for PA leads to better control of blood pressure and improved long-term outcomes. Some series state that up to a quarter of hypertensive patients might have PA, but in routine clinical practice the incidence of PA is much lower.1–4 This discrepancy can be explained by the fact that PA remains a challenging diagnosis which is insufficiently standardised, despite the existence of multiple professional guidelines published during the last decade.5 A recent survey among 33 centres from three continents reported that most centres would use a combination of aldosterone-to-renin ratio as an index for screening, and a saline infusion test for confirmatory testing; however, the cut-off values for these tests showed significant variation among centres.6
Generally, once a diagnosis was made, adrenal vein sampling (AVS) would be performed to confirm laterality, in all patients over the age of 40, who are pursuing surgical treatment for PA.7–10 When bilateral secretion of aldosterone is demonstrated on AVS, patients are offered specific medical treatment with mineralocorticoid receptor antagonists, even though some have demonstrated benefits of adrenalectomy in such patients after resection of the side with the largest aldosterone secretion.11 For unilateral primary aldosteronism, curative unilateral laparoscopic adrenalectomy is the treatment of choice for patients healthy enough, and willing, to undergo an operation. Cross sectional radiological studies (e.g., computerized tomography (CT) scan) are deemed unreliable for confirming the laterality of the adenoma, because of the increasing incidence of small adrenal incidentalomas that could be misinterpreted as the source of excess aldosterone secretion. This limitation of CT scans could be overcome by functional studies positron emission tomography (PET) scans using metomidate, which have proven to be non-inferior to AVS in predicting cure after adrenalectomy for PA in a large study of 143 patient.12 It might be, that with increasing accessibility to the new metomidate PET scanning, the role of AVS in the management of PA could shift, but currently, AVS remains the gold start test prior to proceeding with surgical treatment for PA.
The goal of adrenalectomy for PA is to resolve the excessive aldosterone production, and to cure the hypertension, allowing the discontinuation of antihypertensive medications. The chance of cure of hypertension can be estimated the Primary Aldosteronism Surgical Outcome (PASO) score.13 This score consists of six variables, each of them divided in categories, that are assigned a varying number of prediction points, with a maximum total of 25 points.
Based on the total points, patients are considered more likely to achieve complete clinical success (PASO score>16) or partial/absent clinical success (PASO score≤16). Some of the components of this PASO score are easily available (gender, body mass index, largest adrenal nodule at imaging), while some components are difficult to score in retrospective studies: duration of hypertension is not always recorded accurately, defined daily dose of antihypertensive medications is seldom calculated, and the presence of target organ damage (indicated by micro-albuminuria and/or left ventricle hypertrophy) is not routinely assessed in preoperative tests. For these reasons, the benefits of the score are apparent only in the preoperative discussion with individual patients, rather than in characterising cohorts of patients operated over many years.
Several recent studies suggested that co-secretion of aldosterone and cortisol in patients with Conn’s syndrome is relatively common, with rates as high as 21% when all such patients undergo dexamethasone suppression tests or analysis of their urine steroid profiles.14–19 This would imply that one in five patients who undergo adrenalectomy for PA could be at risk of significant, and potentially life-threatening, cortisol deficiency postoperatively. Such concerns are not confirmed in the surgical practice of most clinicians, and therefore the clinical implications of the alleged Connshing syndrome are yet to be determined.15
Nonetheless, the relatively high rates quoted in some papers might suggest that formal assessment of excessive cortisol co-secretion should be undertaken preoperatively in all patients with PA. This is yet to be routine practice, possibly because overnight dexamethasone suppression test and 24-hour urine steroid profile testing might be considered cumbersome, and an excessive workload, if employed for all patients with PA.
This study aimed to evaluate the ability of AVS to identify patients with co-secretion of cortisol and aldosterone from the same adrenal adenoma, and to thereby select patients who might benefit from a formal evaluation of their hypothalamic-adrenal axis before or immediately after undergoing adrenalectomy for PA.
All patients who underwent an AVS as part of their work-up for surgery for PA between 2014-2022 at a tertiary referral centre were identified from an institutional database. AVS was performed as simultaneous bilateral adrenal venous catheterization, and sampling was performed before and after administration of adrenocorticotropic hormone (ACTH) (250µg Synacten). AVS results were reviewed by the endocrinology team, and patients with bilateral aldosterone secretion were offered medical therapy (hence they were excluded from this analysis). When AVS results were equivocal, the patient was discussed within the endocrinology team, prior to making the decision to refer the patient for consideration of an adrenalectomy. All patients with unilateral secretion were referred for an adrenalectomy, and were formally discussed in a regional Multidisciplinary Adrenal Meeting, attended by endocrine surgeons, endocrinologists, a radiologist, and a pathologist.
Data collection
The study was part of an ongoing institutional audit of outcomes after adrenal surgery (6977/2021). Standard demographics, biochemistry (including results of AVS), operative data and histopathology were collected using an intrahospital database. When more than one blood sample was drawn from the same side, the highest values were recorded.
Adrenal venous sampling procedure
AVS was performed after correction of hypokalaemia and adjustment of the antihypertensive agents, according to current guidelines. All procedures were performed by a dedicated interventional radiology consultant (PB), using sequential cannulation after stimulation with cosyntropin (synthetic adrenocorticotropic hormone 1–24, 250 mcg bolus). This technique is similar to the one used by almost two thirds of the centres surveyed in the AVIS study,20 preferred over the bilaterally simultaneous technique without stimulation. This decision is based on the assumption that the time-difference between blood sampling from one side and the other is negligible, as cortisol secretion is maximally stimulated during cosyntropin infusion. Successful catheterisation and correct position of the catheter tip was confirmed by injection of small amount of contrast with minimal/gentle pressure.
Indices calculated based on AVS results
The Selectivity Index (SI) was calculated by dividing the cortisol concentrations (nmol/L) from each adrenal vein by the cortisol concentration in the infrarenal IVC. As cut-offs for adequacy, SI >2 and >3-5 are generally used with unstimulated, and stimulated sampling, respectively.20,21
The Lateralization Index (LI) was calculated by dividing the ratio of plasma aldosterone concentration (pmol/L) and the plasma cortisol concentration (nmol/L) in the dominant side by the values from the nondominant side. Lateralization indices of >2 for unstimulated and >4 for stimulated sampling are recommended by the Endocrine Society (8). After stimulated AVS, biochemical cure is expected in 81% of patients with LI >4, and in 64% of patients with LI between 2-4 (22). Overall, higher LI is correlated with significantly higher clinical and biochemical success rates after adrenalectomy.22,23
The Contralateral Suppression Index (CSI) was calculated by dividing the ratio of plasma aldosterone concentration (pmol/L) and the plasma cortisol concentration (nmol/L) in the non-dominant side by the values in the infrarenal IVC. This is a helpful adjunctive lateralizing test, as the majority of patients who can be lateralized by using the LI also have a concurrent CSI <1 (24). Patients with CSI <1 have a higher rate of hypertension and biochemical cure (41% and 98%, respectively) compared to those who meet traditional LI criteria but whose CSI is >1 (14% and 56%, respectively).24
Statistical analyses
Summary statistics were obtained, and presented as percentages or median values. Upon comparing categorical data, the χ2-test, or if deemed appropriate Fisher's exact test, was used, while the Mann–Whitney U-test or Kruskal-Wallis-test was used to compare continuous data. Overall, a p-value of less than 0.05 was considered significant. All statistical analyses were performed using StatPlus (AnalystSoft Inc., Version v8).
Patient Cohort
Between June 2014 and November 2022, a total of 92 consecutive patients underwent adrenal surgery for primary hyperaldosteronism, referred based on the results of adrenal venous sampling (AVS). The majority of patients were male (60M:32F), and the average age was 53±11 years. Their average BMI was 30.1±6.3, and the average pre-operative lowest potassium level recorded was 2.79±0.38 mmol/L.
Based on patients’ characteristics, and personal choice of the operating surgeon, laparoscopic adrenalectomy was performed in 32 patients, and retroperitoneoscopic adrenalectomy was performed in 60 patients. The side of the operation was equally distributed (50L:42R). On histological analysis, six patients had adrenal hyperplasia, and the rest had an adenoma, measuring 14±6 mm in maximum diameter.
Results of Adrenal Venous Sampling (AVS)
Median values for SI were 13.2 on the right side (IQR: 8.1-23.2) versus 11.1 on the left side (IQR: 8.1-16.0) (Figure 1). A SI <5 was recorded in 17 right-sided samples, and 10 left-sided samples. A SI <3 was recorded in eight right-sided samples and seven left-sided samples. There was therefore no evidence that the right-side adrenal vein was less likely to be canulated.
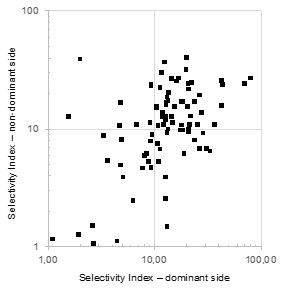
Figure 1 Selectivity Index during adrenal venous sampling in a cohort of 92 patients.
Values for SI were calculated as detailed in Methods. Results are illustrated for dominant side (based on aldosterone secretion) and non-dominant side.
The LI had a median value of 12.9 (IQR: 6.6-29.0), and LI >4 was recorded for all but seven patients. The CSI had a median value of 0.3 (IQR: 0.1-0.7), and 12 patients had CSI >1.
The results from AVS could not predict which patients were found to have adrenal hyperplasia (n=6) rather than an aldosteronoma (n=86). For these two subgroups, there was no statistical difference between the LI (median: 7.5 (IQR: 6.1-10.3) versus 13.5 (IQR: 6.7-30.0); p=0.2), or the absolute values for aldosterone/cortisol ratio on either the dominant side (median: 16.7 (IQR: 7.9-27.3) versus 10.5 (IQR: 5.4-19.1); p=0.5) or on the contralateral side (median: 1.65 (IQR: 0.87-3.4) versus 0.7 (IQR: 0.4-1.28); p=0.06). The CSI was significantly lower in patients with an adenoma (median: 0.3 (IQR: 0.1-0.6)) compared with those with hyperplasia (median: 0.85 (IQR: 0.73-1.13); p<0.01).
Additionally, the absolute values for cortisol concentrations varied widely for both the dominant side (median: 8954 nmol/L (IQR: 5535-12207)), as well as the non-dominant side (median: 6684 nmol/L (IQR: 4458-10547)). There was found to be a poor correlation between these values (r2 0.0721). Moreover, over a quarter of patients were found to have cortisol concentrations that were higher on the contralateral side than the side of the Conn’s adenoma (Figure 2) (Figure 3). Conversely, in thirteen patients (14%), the cortisol concentration on the side of the aldosteronoma was at least 3 time (range 3-32) higher compared with the contralateral adrenal vein, suggesting a degree of predominant secretion on the side of the aldosteronoma. Overall, there was no evidence of inhibition of contralateral secretion of cortisol in any of these patients, as the cortisol levels in the non-dominant side were higher than the peripheral concentration (Figure 4).
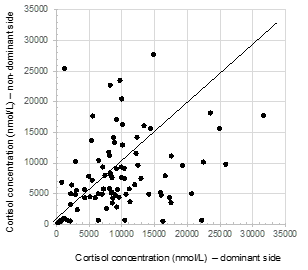
Figure 2 Cortisol concentrations measured in adrenal veins during adrenal venous sampling.
Cortisol concentrations (nmol/L) were measured after ACTH stimulation used routinely in the AVS protocol.
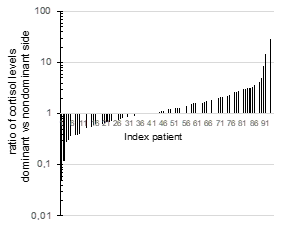
Figure 3 Ratio of cortisol levels in adrenal veins.
A ratio was calculated between cortisol concentration (nmol/L) on the dominant side and non-dominant side in 92 patients who had AVS. The dominant side was considered based on indexes of aldosterone secretion.
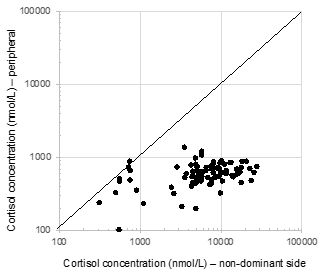
Figure 4 Cortisol concentrations in the adrenal vein on the nondominant side compared with peripheral values.
Postoperative outcomes
None of the patients had cortisol levels checked the morning after their operation, none received cortisol replacement, and none developed clinical signs of adrenal insufficiency. All but one patient were deemed to be cured, based on a reduced need for antihypertensive medication and normal potassium levels within 3-6 months after the operation.
This study comprised data from a cohort of 92 consecutive patients who underwent AVS before undergoing an adrenalectomy for PA. The aim of this analysis was to explore if cortisol-levels measured during AVS could identify patients with Connshing, i.e., patients with excessive secretion of both aldosterone and cortisol from the same adrenal gland/adenoma.
The patients included in this study were considered representative for the population with PA. When compared with a large international cohort of patients with PA characterised in a previous study,23 they had a similar sex and age distribution, but possibly had more severe biochemical abnormalities (the average values for the lowest potassium levels recorded in our cohort were 2.79 mmol/L versus 3.1 mmol/L in the international cohort), and had higher weights (BMI 30.1±6.3 versus 27.6±5.2 in the international cohort).
Data analysis could not demonstrate excess ipsilateral cortisol secretion in any of the patients. Other groups suggested that a larger size of the adenoma could be associated with this phenomenon.16,25,26 The main hypothesis for this, is that the co-secretion of cortisol could simply only become detectable after the tumour has reached a certain size. With an increase in size, the proportion of cortisol-producing cells might increase as well. Moreover, Arlt et al15 reported that an increased degree of urinary glucocorticoid co-secretion was seen more often among those with primary aldosteronism and a higher BMI or insulin resistance, as a possible surrogate of their abnormal glucose metabolism. In our data set, the BMI was not correlated with any of the biological parameters assessed.
The debate about the clinical significance of co-secretion of cortisol in predominantly aldosterone secreting adenomas will most likely continue. Over two decades ago, it was reported that in-vitro assessments suggest that all aldosterone-secreting adenomas co-secrete cortisol to a certain extent,25 as these adenomas are probably more heterogeneous in cell type than purely zona glomerulosa-like cells.27 Furthermore, active conversion from deoxycorticosterone and 18-hydroxy-11-deoxycorticosterone to 18-hydroxycorticosterone and aldosterone, respectively, has been shown to take place in in-vitro tests on adrenal adenoma homogenates from patients with Conn’s adenomas.28
Clinical observations also suggest this is a phenomenon with clinical significance. In a review of published data on 35 patients with aldosterone- and cortisol-co-secreting tumours, almost one-quarter of patients developed adrenal insufficiency.26 Specifically, this occurred in two cases were autonomous cortisol co-secretion was not studied before surgical intervention,29,30 and in six others who experienced severe symptoms of adrenal insufficiency while being on substitution therapy.31–34 It could therefore be hypothesized that the other 75% of patients were in a more subclinical Cushingoid status, due to the co-secretion of aldosterone and cortisol, and that they, therefore, did not develop overt adrenal insufficiency symptoms. The challenge remains how to identify such patients.
The specific hypothesis explored in this study was that excess secretion of cortisol from the side with the aldosteronoma, would inhibit the contralateral adrenal gland, and thus the cortisol levels in the AVS-sample would be found to be much lower on the non-dominant side. The data analysed failed to support this hypothesis. Previously, authors have suggested an unreliable AVS outcome in patients with adenomas that produce both aldosterone and cortisol, as an ipsilateral aldosterone and cortisol co-secreting adenoma may suppress contralateral cortisol secretion, and thus give the impression of an improperly cannulated contralateral vein.26 Conversely, others have reported the opposite, as cortisol co-secreting adenomas may suppress the ipsilateral aldosterone-cortisol ratio while enhancing the contralateral aldosterone-cortisol ratio due to cortisol suppression and thereby falsely suggest bilateral aldosterone hypersecretion.14
The findings from the current study could be influenced by the stimulation with ACTH, which all patients received during the AVS procedure, and this could have ‘masked’ any (partial) inhibition allegedly triggered by a Connshing adenoma. It thus remains possible that without ACTH stimulation, this phenomenon might have been demonstrated, and a similar analysis performed in a centre where AVS is done without ACTH stimulation might lead to different results.
The strength of this study is that it describes a rather typical, unselected population of patients with Conn’s adenoma who had successful AVS facilitating effective treatment of their condition. Moreover, the patients included were all worked-up, and operated on, in a single tertiary centre, and were thus subjected to a uniform AVS-protocol. The number of patients included, should have been sufficient to demonstrate a potential inhibited cortisol secretion from the contralateral adrenal among those patients with excess secretion of cortisol from the side with the aldosteronoma, based on the available incidences in literature.
A significant limitation of the study, is that no formal pre- or post-operative assessment of the cortisol secretion was undertaken. The uneventful recovery, and the absence of the need for post-operative steroid replacement, were considered as a surrogate for appropriate postoperative adrenal function in all patients. A second limitation was the reliance on historical histopathology reports, based on microscopic appearance of adrenal nodules without functional characterisation. Recent international guidelines and from the Royal College of Pathologists, recommend formal immunohistochemical analysis of all specimens resected from patients with PA,35,36 to demonstrate specific expression of enzymes involved in the synthesis of aldosterone and cortisol and future histopathology reports might bring a new insight into this debate. Another, potential, limitation could be that the focus of this analysis was only on the immediate perioperative events rather than on the long-term follow-up of this large cohort of patients with PA.
Based on an international consensus on outcome measures after adrenalectomy for PA, patients with complete clinical success are those with postoperative normotension (i.e., SBP <140 mmHg and DBP <90 mmHg) without the aid of antihypertensive medication.23 Using a large international cohort of 705 patients operated in 12 referral centres in 9 countries, the authors reported that complete clinical success was achieved in 37% patients, with a wide variance between centres (range 17–62%), and partial clinical success in an additional 47% (range 35–66%), while complete biochemical success was seen in 94% patients. Similarly, we reported that in a group of 38 patients operated in our unit between 2005-2012, and followed up for a median of 30 months, 85% of them were taking less antihypertensive medication37, but the exact information has not been collected in this more recent cohort. As this study was recorded as an audit for service evaluation, there were no additional clinical appointments made for patients, and information would have been incomplete for many of our patients who were operated as extraterritorial referrals.
This study showed that biochemical assessment of samples from AVS cannot identify patients with co-secretion of aldosterone and cortisol. Therefore, cortisol-levels during AVS cannot be used to identify patients who might benefit from a formal perioperative assessment of the hypothalamic-adrenal axis.
None.
Authors declare that there is no conflict of interest exists.
None.

©2023 de, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.