eISSN: 2473-0815


Review Article Volume 6 Issue 4
Rajiv Ghandhi University of health science, India
Correspondence: Sreelatha S, Professor, Rajiv Ghandhi University of health science, India, Tel 9448915477
Received: January 19, 2018 | Published: July 10, 2018
Citation: Sreelatha S, Kamala R, Nadagoudar S, et al. A clinical review of obstetric and perinatal outcome in thyroid disorders. Endocrinol Metab Int J. 2018;6(4):266-282. DOI: 10.15406/emij.2018.06.00188
Thyroid disorders are the second most common cause of endocrine dysfunction in women of child bearing age after diabetes mellitus. It is now well established that not only overt, but also subclinical thyroid dysfunction have significant adverse effects on pregnancy and fetal development. The adverse pregnancy outcomes include, miscarriage, pregnancy induced hypertension, and its more severe form pre-eclampsia, as well as placental abruption, anaemia, post partum hemorrhage, and increased fetal morbidity and mortality. Thyroid testing is a must at first booking. Adequate replacement therapy should be done to keep TSH with in trimester specific referrence ranges. Early and effective treatment of thyroid disorders ensures safe pregnancy with minimal maternal and fetal complication.
Keywords: hypothyroidism, hyperthyroidism, pregnancy, anemia, PRE eclampsia
Thyroid disorders are the second most common cause of endocrine dysfunction in women of child bearing age after diabetes mellitus.1–3 Development of maternal thyroid disorders during early pregnancy can influence the pregnancy outcome and fetal development. It is now well established that not only overt, but also subclinical thyroid dysfunction have significant adverse effects on pregnancy and fetal development. The adverse pregnancy outcomes include, miscarriage, pregnancy induced hypertension, and its more severe form pre-eclampsia, as well as placental abruption, anaemia, post partum hemorrhage, and increased fetal morbidity and mortality. These obstetric complication contribute to overall increase in the frequency of adverse neonatal outcomes, which include preterm birth, low birth weight, increase admission to neonatal intensive care and increase perinatal morbidity and mortality.4–9 Iodine deficiency significantly raises the risk of still birth and abortion amongst pregnant women and also leads to decreased availability of iodine to the fetus. It retards the neurological development in fetus stage and also impairs the cognitive development thereby leading to learning disability and lowered achievement motivation in later stages of childhood.10,11 Hyperthyroidism in pregnancy is less common than hypothyroidism. Untreated hyperthyroidism during pregnancy is associated with maternal and fetal morbidity. Neonatal Graves’s disease can be seen because of the passage of TRAb to the fetus from the mother and may be seen in about 1-5% of the babies.12
Thyroid gland
The thyroid gland is an endocrine gland first described by Thomas Wharton [1616-1673] of England. The word thyroid is derived from Greek words (“thyreos”-sheid, plus “eidos”-form). It weighs normally between 12-20gms. It has two lobes that are connected by an isthmus. It is wrapped around the trachea as though it is a shield for the trachea. It is highly vascular, and soft in consistency. Enlargement of thyroid gland is called goiter. Toxic goiter secretes excess thyroid hormones. Non- toxic goiter secretes normal or even subnormal levels of hormones.13,14 The thyroid gland develops from the floor of primitive pharynx during third week of gestation. The gland migrates from the foramen cecum, at the base of the tongue, along the thyroglossal duct to reach its final location in the neck. This feature accounts for the rare ectopic location of thyroid tissue at the base of the tongue (lingual thyroid), as well as for the presence of thyroglossal duct cysts along the developmental tract.13 Thyroid gland development is controlled by coordinated action of developmental transcription factors. Thyroid transcription factor (TTF-1) also known as NKX2A, TTF-2(also known as FKHL15), and paired homeobox (PAX-8) are expressed electively, but not exclusively, in the thyroid gland. In combination, they dictate thyroid cell development and induction of thyroid specific genes such asthyroglobulin (Tg), thyroid peroxidase (TPO), the sodium iodide symporter (NIS) and the thyroid stimulating hormone receptor (TSH-R).13 Mutation in these developmental transcription factors or their downstream target genes are rare causes of thyroid agenesis or dyshormonogenesis and cause congenital hypothyroidism.13
Microscopic features
The mature thyroid gland contains numerous spherical follicles. The average diameter of the follicle is 200µm. Each follicle is lined by single row of cells called follicular cells that surround the secreted colloid. Colloid is a proteinaceous fluid that contains large amount of throglobulin (Tg), the protein precursor of thyroid hormones. Tg is a protein and thyroid hormones are obtained from Tg. When the gland is inactive the colloid is abundant, the follicles are larger the cells lining them are flat.13,14 When the gland is active, the follicles are small, the cells are cuboidal or columnar and the areas where colloid is being actively reabsorbed in to thyrocytes are visible as “resorption lacunae”. Microvilli project into the colloid from the apices of the thyroid cells and canaliculi extend into them. The endoplasmic reticulum is prominent, a feature common in most glandular cells. The Thyroid follicular cells are polarized - the basolateral surface is opposed to the blood stream and an apical surface faces the follicular lumen. Increased demand for thyroid hormone usually signaled by thyroid stimulating hormone (TSH) binding to its receptor on the basolateral surface of follicular cells, leads to Tg resorption from the follicular lumen and proteolysis within the cell to yield thyroid hormones for secretion in to the blood stream.13–15 The thyroid gland is highly vascular. The thyroid gland has a blood flow about five times the weight of the gland each minute. It receives both sympathetic and parasympathetic nerve supply. Sympathetic nerves control the blood supply of the thyroid gland (Figure 1).14
The thyroid hormones-chemistry
Thyroid gland secretes:
Iodine is required for the formation of thyroxine: To form normal quantities of thyroxine, about 50 mg of ingested iodine in the form of iodides are required each year, or about 1mg/week. To prevent iodine deficiency, common table salt is iodized with about 1 part sodium iodide to every 100,000 parts sodium chloride.16
Fate of ingested iodides: Iodides ingested orally are absorbed from the gastro-intestinal tract into the blood in about the same manner as chlorides. Most of the iodides are rapidly excreted by the kidney, but only after about one fifth are selectively removed from the circulating blood by the cells of the thyroid gland and used for synthesis of thyroid hormones.16
Iodide trapping (Iodide pump): Food iodide from the blood is taken up by the follicular cells of thyroid-a process called iodide trapping. Iodide trapping occurs against electrochemical gradient because the interior of the follicular cells is –ve and iodide ion is also –ve. The iodide concentration of follicular cells is 30 times higher than in the blood hence, iodide trapping is a active process requires the activity of Na+K+ATPase. When the thyroid gland becomes maximally active, this concentration ratio can rise to as high as 250 times.14–16 The rate of iodide trapping is influenced by several factors, the most important being the concentration of TSH; TSH stimulates and hypophysectomy greatly diminishes the activity of iodide pump in thyroid cells.16
Thyroid hormone biosynthesis oxidation of iodide ion: The first essential step in the formation of the thyroid hormones is conversion of iodide ions to an oxidized form of iodine that is then capable of combining directly with amino acid tyrosine. This oxidation of iodine is promoted by the enzyme peroxidase and its accompanying hydrogen peroxide, which provides potent system capable of oxidizing iodides. The peroxidase is either located in the apical membrane of the cell or attached to it, thus providing the oxidized iodine at exactly the point in the cell where the thyroglobulin molecule issues forth from the golgi apparatus and through the cell membrane in to the stored thyroid gland colloid. When the peroxidase system is blocked or when it is hereditarily absent from the cells, the rate of formation of thyroid hormone falls to zero.16
Iodination of tyrosine and formation of thyroid hormones-“organification of thyroglobulin”
The binding of iodine to thyroglobulin molecule is called as organification of the thyroglobulin. The oxidized iodine is associated with an iodinase enzyme that causes the process to occur within seconds or minutes. Tyrosine is first iodized to monoiodotyrosine and then to diiodotyrosine. Then iodotyrosine residues become coupled with one another.16 The major hormonal product of coupling reaction is the molecule thyroxine that remains part of thyroglobulin molecule or one molecule of monoiodothyrosine couples with one molecule of diiodotyrosine, which represents about one fifteenth of final hormones.16
Storage of thyroglobulin
The thyroid gland is unusual among the endocrine glands in its ability to store large amounts of hormone. After synthesis of thyroid hormones has run its course, each thyroglobulin molecule contains up to 30 thyroxine molecules and a few triiodotyrosine molecules. In this form the thyroid hormones are stored in the follicles in an amount sufficient to supply the body with its normal requirements of thyroid hormones for 2 to 3 months. Therefore, when synthesis of thyroid hormone ceases, the physiologic effects of deficiency are not observed for several months.16
Release
Thyroglobulin itself is not released in to the circulating blood in measurable amounts, instead, thyroxine and triiodotyronine must first be cleaved romthyroglobulin molecule, and then these free hormones are released. This process occurs as follows: the apical surface of the thyroid cells send out pseudopod extensions that close around small portions of colloid to form pinocytic vesicles that enter the apex of the thyroid cell. Then lysosomes in the cell cytoplasm immediately fuse with these vesicles containing digestive enzymes from the lysosomes mixed with the colloid. Multiple proteases among the enzymes digest the thyroglobulin molecules and release thyroxine and triodothyronine in free forms. These are then released in to the blood.16 About three quarters of the iodinated tyrosine in the thyroglobulin never becomes thyroid hormones but remains the same and they are not secreted in to the blood. Instead their iodine is cleaved from them by deiodinase enzyme that makes virtually all this iodine available again for recycling within the gland for formation of additional thyroid hormones.16 In the congenital absence of this deiodinase enzyme, it makes the person iodine deficient because of failure of the recycling process (Figure 3).16
Transport in the blood
Over 99% of T4 and T3 are bound to plasma proteins and less than 1% is unbound (free). But this unbound fraction alone can combine with their receptors to perform the thyroid hormone functions and subsequently degraded. The bound fraction serves as reservoir. When the free fraction is diminished, a portion of the bound fraction becomes unbound to replenish the free fraction. Three plasma proteins, all synthesized in the liver bind with iodothyronines:14–16
Mechanism of action of thyroid hormones at molecular level
Virtually all cells of the body are target cells of thyroid hormones. Both T4 & T3 can do enter the target cell by crossing the cell membrane. Within the cell most of the T4 is converted to T3. After injection of large quantities of thyroxine into the human being, essentialy no effect on the metabolism is discerned for 2 to 3 days, thereby demonstrating that there is a long latent period before thyroxine activity begins. Once activity does begin, it increases progressively and reaches a maximum in 10 to 12 days, thereafter it decreases with half life of about 15days. Some of the activity persists for as long as 6weeks to 2months.16 The actions of T3 occurs about four times more rapidly as those of T4, with a latent period as short as 6- 12 hours and maximal cellular activity occurs in 2-3 days. T3 is however generated and modulated by a group of three selenoprotein enzymes. T4 requires mono-de-iodination of the outer ring of the iodothyronine molecule to produce T3, the active metabolite. Type 1 and 2 de-iodinase enzymes are able to do this. Type 1 de-iodinase is responsible for production of most of the peripheral circulating T3 and is expressed predominantly in the liver and kidney. Type 2 DI activities is important for the local tissue supply of T3 and is found in the brain, brown adipose tissue and pituitary. Conversely, type 3 DI is responsible for inner ring de-iodination, which converts T4 to reverse T3 (rT3) and T3 to di- iodothyronine (T2), both of which are inactive metabolites.17 Type 3 DI activity is stimulated by T3, so T3 concentrations can be regulatedlocally. There is an abundance of this enzyme in trophoblast, which accounts for the high circulating levels of rT3 in the fetus. The specific role for rT3 in humans is unclear. In rodents, rT3 has been shown to stimulate adipocyte metabolism, amino acid uptake, hepatic amino-transferase and growth hormone secretion. T4 and T3 can also be reversibly conjugated with glucuronide and sulphate, with excretion via bile or urine which allows the retention of iodine. Conjugated T3has no affinity for thyroid receptors and very low levels are found in the adult. The T3 combines with the receptors situated on the nucleus, this combination causes some genes to be activated which leads to transcription of mRNA leading to synthesis of protein enzymes, structural proteins, transport proteins and other substances.16,17
Physiologic functions of thyroid hormones
Effect of thyroid hormone on growth
For growth and maturation thyroid hormones are necessary. For this to occur, the action of T4 & T3 is helped by Insulin growth factor and growth hormone. For maturation of growth centres, T4 & T3 are required even in fetal life. Thyroid hormones also stimulates the process of bone remodeling. For normal functioning of skeletal muscles, thyroid hormones are required.14
Effect on the CNS
For proper development of brain, presence of T4 & T3 is essential in the fetal brain during infancy. T4 & T3 are required for growth of cerebrum, cerebellum, proliferation and branching of nerve fibers, along with myelination. The fiber branching requires the presence of NGF [nerve growth factor]. If thyroid deficiency is not corrected within few months of birth, brain deficiency leads to cretinism [i.e. cannot be corrected by administration of thyroid hormones afterwards]. The hyperthyroid individual is likely to have extreme nervousness and many psychoneurotic tendencies, such as anxiety complexes, extreme worry & aranoia.16
Effect on sexual function
Lack of the thyroid hormone in males is likely to cause loss of libido, defects in spermatogenesis; great excess of hormone, however sometimes cause impotence. In the females, lack of thyroid hormone often causes menorrhagia, polymenorrhoea, irregular menses, and amenorrhoea. They are also required for follicular development ovulation as well as for proper progress of pregnancy.14–16
Effect on specific bodily mechanisms
Stimulation of carbohydrate metabolism:
Stimulation of fat metabolism
Thyroid hormone mobilizes lipids rapidly from fat tissue, which decreases the fat stores in the body, this also increases the free fatty acid concentration in the plasma and greatly accelerates the oxidation of free fatty acids by the cells.16 Effect on plasma and liver fats:
Effect on sleep
Because of the exhausting effects of thyroid hormone on the musculature and on CNS, the hyperthyroid subject often has a feeling of constant tiredness. But because of the excitable effect of thyroid hormones on the synapses, it is difficult to sleep. Conversely extreme somnolence is a characteristic of hypothyroidism, with sleep sometimes lasting from 12 to 14 hours a day.16
Effect on cardiovascular system
Effect on sympathetic system
Catecholamines [adrenaline & noradrenaline] enhance
Thyroid hormone facilitates all the three actions of catecholamines. Thyroid hormones increase the β1 adrenergic receptors of heart, hence in thyrotoxicosis catecholamine response to heart [eg: tachycardia, palpitation] is enhanced, therefore in thyrotoxicosis along with anti-thyroid drugs β-blockers are used.14–16
Defect on the function of the muscles
Slight increase in thyroid hormone usually makes the muscle react with vigor, but when the quantity of hormone becomes excessive, the muscles become weakened because of excessive protein catabolism. Conversely lack of thyroid hormones causes the muscles to become sluggish, they relax slowly after a contraction.16
Effects on gastrointestinal motility
Hyperthyroidism often results in diarrhoea and Hypothyroidism often results in constipation.13
Control of thyroid secretion
Secretion of the thyroid hormones are depends upon two major factors
HPT Axis
Median eminence of hypothalamus secretes TRH [thyrotropin releasing hormone], a bipeptide with molecular weight 28,000daltons.14–16 TRH stimulates thethyrotrophs of anterior pituitary to secrete and release TSH. Stimulates the follicular cells of thyroid gland and stimulates every step of thyroid hormone synthesis. The ability of TSH to trap iodide depends to some extent on the blood iodide concentration. If the concentration of blood iodine is high [eg: high iodine intake], despite the presence of adequate TSH, iodide trapping by follicular cells is poor.13,14,16 If the food iodine intake is very low, TSH causes sharp iodide trapping.
Negative feedback
If food iodine content is very low, little or no T4 is formed, owing to –ve feedback mechanism, TSH secretion increases leading to goiter and this condition is called as iodine deficiency goiter. When the serum concentration of T4 & T3 is very low [hypothyroidism], the serum concentration of TSH, owing to the –ve feedback mechanism should be very high. Conversely, where there is high T4 & T3 in the serum, the TSH concentration of serum should be very low or nil [hyperthyroidism].13–16
Effects of TSH:16
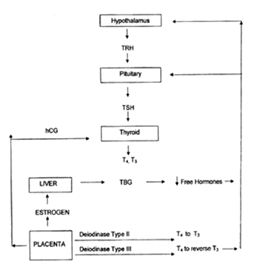
Figure 4 Schematic Representation of the Physiologic Adaptation to Pregnancy, showing increased Thyroxine Binding Globulin (Tbg) Concentrations, Increasedhuman Chorionic Gonadotropin (Hcg) with its Thyrotropin-like activity, Andalterations in Peripheral Metabolism of Thyroid Hormones in Theplacenta.
Physiology of thyroid in pregnancy
In early pregnancy, materal thyroid gland is challenged with an increased demand for thyroid harmone secretion, due mainly to three different factors:
The normal thyroid gland is able to compensate for increase in thyroid harmone demands by increasing its secretion of thyroid harmones and maintaining them within normal limits throughout gestation. Active secretion of thyroid harmones by fetal thyroid gland commences at about 18 weeks of gestation, although iodine uptake by fetal gland occurs between 10 and 14 weeks. Transfer of thyroxine (T4) from mother to embryo occurs from early pregnancy.
Maternal T4 has been demonstrated in coelomic fluid at 6 weeks and in the fetal brain by 8 weeks gestation supporting the important role of maternal thyroid harmone during first trimester of pregnancy for fetal brain development. Several studies suggested that mild maternal thyroid harmone deficiency in the first trimiser could result in long-term neurophysiological damage to the offspring. Despite these acute changes in total harmone concentration, the serum free fraction of both T3 and T4 remains within normal limits, unless there is decreased supply of iodine to the mother or in the presence of abnormalities of thyroid gland. Goiter is commonly seen in pregnancies in areas with iodine deficieny, however in the United States and other areas of the world with sufficient iodine intake, the thyroid gland doesn’t clinically increase in size during pregnancy. Therefore, the detection of a goiter in pregnancy is an abnormal finding that needs careful evaluation. The most common cause of diffuse goiter is chronic auto immune thyroiditis or Hashimoto thyroiditis. Most patients are euthyroid, and his diagnosis is made by the determination of thyroid antibodies, mainly thyroid peroxidase (TPO). Antibody concentration decreases in pregnancy and increases in post partum period. Higher values in first trimester of pregnancy are predictors of syndrome of post partum thyroid dysfunction. In pregnancy, fetus receives iodine from maternal source in all the trimisters. Fetus receives thyroxine from mother up to 12 weeks through placental circulation but not TSH or ft3. Thyroid gland is the first of the endocrine gland to develop, on approximately 24th day of gestatation. Thyroxine is partially converted to ft3 and combines with receptors in fetal brain and responsible for fetal brain development. From 12th week, placental changes resist T4 passage to fetus and fetal pitutary thyroid axis starts functioning like adult.
Iodine in pregnancy and after delivery
Iodine metabolism
Iodine metabolism in pregnancy is marked by several characteristics. Synthesis of thyroid hormones is increased by up to 50% due to estrogen induced increase in TBG concentration. Renal clearance of iodide increases owing to higher glomerular filtration rate. Iodide & iodothyronines are transported from maternal circulation to the fetus20. Fetal thyroid hormone production increases during second half of gestation and after delivery. Iodide is also transported in to the breast milk.20 Iodine supply:
According to Endocrine Society Clinical Practice Guidelines,
Maternal thyroid function
Transport protein
Besides TBG, which is the major thyroid hormone transporter proteins, transthyretin and albumin are also important. The level of albumin, which has lowest thyroxine affinity and enables a fast release of T4, gradually decrease during pregnancy.20,23 TBG is an active carrier and has a possibility to switch between high affinity low affinity forms.24 The TBG levels are highest in the second and third trimester of pregnancy22 and the same holds true for thyroid hormone binding ratios25 and thyroid binding capacity,26 which decreases as soon as 3-4days after delivery. In pregnancy TBG production in the liver is increased and half life of TBG is prolonged because of estrogen induced increase in sialylation on TBG, therefore t1/2 is increased from 15min to 3days.20
Variations in HCG
HCG has intrinsic thyrotropic activity. It increases shortly after conception, peaks around gestational age week 10, declines to a nadir by week 20.20 It directly activates TSH receptor. Transient decrease in TSH between 8-14wks mirrors the peak HCG concentration. In 20% of normal women TSH level decreases to less than lower limit of normal. During the first trimester of pregnancy, when hCG is at its greatest concentration, serum TSH concentrations drop, creating the inverse image of hCG. In most pregnancies, this decrease in TSH remains within the health-related reference interval.22 Under pathological conditions in which hCG concentrations are markedly increased for extended periods, significant hCG-induced thyroid stimulation can occur, decreasing TSH and increasing free hormone concentrations. Members of the glycoprotein hormone family of luteinizing hormone, follicle-stimulating hormone, TSH, and hCG contain a common α-subunit and a hormone-specific β-subunit. Because the hCG and TSH β-subunits share 85% sequence homology in the first 114 amino acids and contain 12 cysteine residues at highly conserved positions, it is likely that their tertiary structures are very similar.27,28 Purified hCG, like TSH, has been shown to:
It has estimated that a 10,000IU/L increment in circulating hCG corresponds to a mean free T4 increment in serum of 0.6 pmol/L (0.1 ng/dL) and in turn, to a lowering of serum TSH of 0.1 mIU/L.20 Hence, it is predicted that an increase in serum free T4 during the first trimester will be observed only when hCG concentrations of 50,000–75,000 IU/L are maintained for >1week.28 Some patients may be oversensitive to circulating hCG. Recently, it had been described in two patients, a mother and her daughter, with recurrent gestational hyperthyroidism and severe nausea, despite serum hCG concentrations within the health-related reference interval.29 Both women were heterozygous for a missense mutation in the extracellular domain of the thyrotropin receptor. The mutation, a substitution of guanine for adenine at codon 183, led to the replacement of a lysine residue with an arginine (K183R). When expressed in COS-7 cells, the mutant receptor was 30-fold more sensitive than the wild-type receptor to hCG, as measured by cAMP production. The mutation thereby could account for the occurrence of hyperthyroidism in these two women despite the presence of hCG concentrations within the reference interval. Further studies are needed to determine the incidence of this mutation in the general population.28
Variations in TSH
Most authors agree that in the first trimester, TSH levels may be decreased in some women with otherwise healthy thyroid gland. During pregnancy, TSH level increases and reach the highest value in the third trimester, irrespective of iodine supply.22 After 3-4days after delivery, TSH levels were the highest.26 Higher TSH levels in second half of pregnancy probably mirrors HCG and free thyroid hormones levels, being lower in that period of pregnancy. A total of 4months after delivery, TSH level were lower than in third trimester.30 After 1 yr postpartum they were lower than in the second and third trimester (Figure 5).20
Thyroid function tests during pregnancy
Variations in free thyroid hormones
Even in areas with adequate iodine intake, many authors established pregnancy levels of the fT4 and fT3 to be lower than in non-pregnant individuals. In the last months of pregnancy, fT4 levels were often below the reference interval. Free thyroxine slightly increased in the first trimester and decreased by approximately 30% to low normal values in second and third trimester.31 Several factors may influence the level free thyroid hormones. Increased hCG at 11-13wks was associated with increased median values of fT4.32 Twin pregnancies with higher hCG values of longer duration frequently lead to increased fT4. 20 Normal FT3 level is 1.7 to 4.2 pg/ml and FT4 level is 0.7 to 1.8 ng/ml.
Maternal thyroid size
Thyroid size is influenced by different factors, including iodine supply, genetics, gender, age, TSH, anthropometric parameters, parity and smoking.33 In areas with adequate iodine intake, thyroid volume did not change during pregnancy.34 Increase in thyroid volume during pregnancy was followed by the decrease after delivery and on the basis of this finding it was postulated that increased vascularity may be the reason for the increase in thyroid volume.20 Increased thyroxineinding globulin increases serum thyroxine (T4) concentrations, and chorionic gonadotropin has thyrotropin-like activity and stimulates maternal T4 secretion.
The transient hCG induced increase in serum T4 levels inhibits maternal secretion of thyrotropin except for minimally increased free T4 levels when hCG peaks, these levels are essentially unchanged (T3 = triiodothyronine) (Figure 6).

Figure 6 Relative changes occur in maternal thyroid function during pregnancy aternalchanges include a marked and early increase in hepatic production of thyr oxine-binding globulin (TBG) and placental production of chorionic gonadotropin (hCG).
Thyroid autoimmunity in pregnancy & after delivery
The role of pregnancy in triggering of thyroid autoimmunity
Immune adaptations in pregnancy
In order to tolerate the fetus during the intrauterine life, the mother’s immune system undergoes several adjustments. Both maternal systemic suppression and placental immune suppression are involved in preserving the pregnancy, being induced by significant hormonal changes. The key regulatory role is carried out by regulatory CD4+CD25+T cells (Treg) being important not only in peripheral tolerance against both foreign and self-antigens, but also in fetal tolerance. Treg cells were shown to regulate both Th1-type activity, which leads to cellular immunity, and Th2-type activity, being involved in humoral immunity.35 In pregnancy, the expansion of treg cells is presumably provoked by fetal antigen presentation and estrogen-induced expression of several chemokines. They occur in early pregnancy and they increase rapidly during pregnancy, peaking in the second trimester.20 Treg cells, accumulated predominantly in decidual tissue and to a lesser extent in peripheral blood,36 were shown to significantly suppress both Th1-type and Th2-type reactions against paternal/fetal allo-antigens. However, Th2 clones seem to be less sensitive to this suppression than Th1 clones, leading to predomination of Th2 cells and cytokines over a Th1 cellular Treg cells immune response, driving the cytokine balance away from the detrimental effects of Th1-cell activity, which may cause fetal loss.37 Therefore, the maintenance of pregnancy is enabled by proper balance of Th1/Th2 immunity, with a slight shift towards Th2 immunity. This physiological state of lowered immune responsiveness in pregnancy results in amelioration of some pre-existing autoimmune disorders, such as rheumatoid arthritis, multiple sclerosis or thyroid autoimmune disease.38 During the weeks immediately prior to delivery a clear decline in Treg cells occurs. After delivery, this imbalance in Treg cells and shift of cytokine profile away from Th2 to Th1 during the return to normal pre-pregnancy state may be reflected in exacerbation or aggravation of autoimmunity.39 In pregnancy and postpartum, different types of autoimmune thyroid disease may occur, including Graves’ disease (GD), Hashimoto’s thyroiditis (HT) and postpartum thyroiditis (PPT). Characteristically, thyroid autoantibodies decline during pregnancy, which might be explained by the Treg-mediated suppression. After delivery, they return to the pre-pregnant values, frequently ending in postpartum exacerbation of thyroid autoimmunity40 (Figure 7).
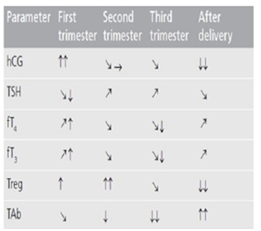
Figure 7 Thyroid physiology and autoimmunity in pregnancy and after delivery.
→: No change; _: Slight decrease; ↓: Decrease; ↓↓: Marked decrease; _: Slight increase; ↑: Increase; ↑↑: Marked increase when compared with the previous period. fT3: Free triiodothyronine; fT4: Free thyroxine; hCG: Human chorionic gonadotropin; TAb: Thyroid autoantibodies; TSH: Thyroid stimulating hormone.
The role of fetal microchimerism
Fetal microchimerism refers to the phenomena of fetal cell leakage into the mother’s circulation through the placenta during pregnancy. The presence of chimeric male cells has been established in the peripheral blood and maternal tissues, including thyroid,41 and they have been found circulating in mothers several years after delivery.42
In Grave’s Disease and Hashimoto’s Thyroiditis, intrathyroidal fetal microchimeric cells were detected significantly more often than in non autoimmune thyroid disease20 however, large population-based studies found no association between parity and thyroid autoimmunity, arguing against a key role of fetal microchimerism43
Female sex
Several large epidemiological studies confirmed the female predominance in thyroid autoimmunity, as they present with positive thyroid autoantibodies approximately two- to three-times more often than males.44 Estimation based on the largest National Health and Nutrition Examination Survey (NHANES) III study, indicated that 17% of females were positive for thyroid peroxidase antibodies (TPOAb), while 15.2% were positive for Tg antibodies (TgAb). Additionally, the prevalence of antibodies was twice as high in white females compared with black females.44 Besides fetal microchimerism, higher genetic susceptibility for thyroid autoantibody production in females than in males has been reported in the study of Danish twins.45 X chromosome genes are essential in determining sex hormone levels, as well as in maintaining immune tolerance. Therefore, the alterations in X chromosome, including monosomy or structural abnormalities, and disturbances in X chromosome inactivation with consequent impaired thymic deletion of auto reactive cells, might contribute to the impaired immune response20
Risk predisposing factors
Genes
Appropriate genetic background is needed to allow different endogenous and environmental influences to trigger thyroid autoimmunity. Initial observations of higher incidence of thyroid autoimmune disease in families have been recently confirmed by two reports, showing that risk for developing thyroid autoimmune dis- ease was around 16-fold increased in children and siblings of the affected individuals.46 According to the estimation based on Danish twins, genetic influence seems to contribute 73% to thyroid autoantibody positivity.45 Until now, several putative genes have been identified. Among immune regulatory genes, HLA-DR gene, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) gene, CD40 gene, protein tyrosine phosphatase-22 (PTPN22) gene and CD25 gene have shown an association with thyroid autoimmune disease. Among thyroid-specific genes, major candidates are the gene for Tg and TSH receptor gene.47 Besides being involved in clinical disease, genetic susceptibility is crucial also for thyroid antibody production. Among putative genes, CTLA-4 was confirmed as a major locus for thyroid antibodies,48 being associated with higher thyroid antibody levels in Grave’s Disease, Hashimoto’s Thyroiditis and Post partum Thyroiditis.49
Iodine intake
The enhancing influence of iodine on thyroid autoimmunity has been confirmed by studies on experimental animal models and also by large observational studies of populations with different iodine intake. Among mechanisms, autoantigenic potency of highly iodinated Tg or iodine toxicity to thyrocytes have been proposed, but the precise mechanism is still unknown.50 In humans, the improvement of the iodine prophylaxis lead to a three–fourfold increase in incidence of thyroid autoimmunity in a population with previously mild iodine deficiency.51 The prevalence of thyroid antibodies, estimated by large epidemiological studies, was up to 25% in conditions of excessive iodine intake.52 High iodine intake in pregnancy was associated with a higher risk of developing PPT,53 but this observation was not supported by other studies showing that iodine supplementation in pregnancy and after delivery is safe even in TPOAb-positive females54
Other risk factors
Other risk factors, although less frequent in pregnancy and postpartum, might contribute to thyroid autoimmunity in females in the reproductive period. Smokers are at risk for Grave’s Disease55 and at even greater risk for either development or deterioration of Graves’ orbitopathy.56 In Hashimotos Thyroiditis, few early investigations implicated the association with smoking,57 while a recent report indicated even negative relation of smoking with both TPOAb and TgAb as well as with hypothyroidism.52 Also, data regarding Post Partum Thyroiditis are scarce, with only two studies implying the increased risk in association with smoking.52 Triggers such as stress, infections, environmental toxicants or immune-modulating drugs may contribute to thyroid autoimmunity in the reproductive period equally as in the general population.59
Hypothyroidism
Incidence of Hypothyroidism is 2.5%.20Symptoms of hypothyroidism are often masked by hypermetabolic state of pregnancy. Subclinical hypothyroidism means increase in TSH with normal fT3 &fT4.20 Overt hypothyroidism means increase in TSH with decrease in fT3 & fT4.20 Untreated hypothyroidism is associated with pregnancy induced hypertension, abruption placenta, postpartum hemorrhage, premature birth, low birth weight infants and impaired neurodevelopment in offspring.28
American thyroid association (2007) recommends cut off values for TSH as,
Etiology of hypothyroidism in pregnancy20,28
Diagnosis of hypothyroidism
Clinical signs and symptoms28
Cold intolerance
Laboratory assessment of hypothyroidism must be made using TSH and free hormone levels assessment. Total T3 &T4 measurments should be considered unreliable because of increase in TBG concentration. Anti TPO antibodies and anti thyroglobulin antibodies are increased in most patients of hashimotos thyroiditis & therefore helps in diagnosis. In addition pregnant women who are on thyroid replacement therapy require larger doses compared to no pregnant patients because of increase in TBG concentration & increase in type 3 deiodinases from the placenta. TSH should be monitored closely and the doses of thyroid replacement to be adjusted to maintain TSH in reference interval. Doses of thyroid replacement therapy can be lowered to prepregnancy levels at parturition.28 The starting dose of levothyroxine is 1-2µg/kg/day. It should be adjusted every 6-8 weeks to keep TSH at lower end of normal. Women who are on levothyroxin at the beginning of pregnancy should have their dose increased approximately 30% as soon as pregnancy is confirmed. Levothyroxine should be taken orally on empty stomach (45 minutes before consumption of food,beverages, or other medications). In addition Ferrous sulfate and calcium and pre natal doses should be avoided within 4 hours of ingestion of LT4, as theses can decrease the absorption of thyroxine. Our aim is to keepthe free thyroxine value in the upper normal range.60
Follow up after delivery
After delivery, levothyroxine therapy should be returned to prepregnancy dose, and TSH checked 8wks postpartum. Breastfeeding is not contraindicated in women treated for hypothyroidism. Levothyroxine is excreted in breast milk, but the levels are too low to alter thyroid function in the infant. Periodic monitoring with an annual serum TSH concentration for the mothers is generally recommended.61 Causes of Raised TSH Activity in Patients Receiving Standard Replacement Doses of LT62
Non–compliance–supervised administration of standard daily or single weekly dose of 1000g
Journal of thyroid research concordant with American thyroid association guidelines gives the following recommendations63
Hyperthyroidism
Incidence of Hyperthyroidism is 0.2%.28 Subclinical hyperthyroidism is defined as serum TSH concentration below the lower limit of reference range, with fT3 & fT4 concentration within normal range.28 Overt hyperthyroidism is defined as serum TSH concentration below the lower limit of reference range, with increase in fT3 & fT4 concentration.28 Gestational transient hyperthyroidism is associated with hyperemesis gravidarum commonly presenting with elevated levels of fT4 and suppressed levels of TSH.28 This change is associated with β-HCG stimulation of thyroid gland. Fetus of hyperthyroid mother is at risk because; TSH receptor stimulating autoantibodies are the culprits of pathogenesis in the fetus. The likelihood of developing fetal hyperthyroidism requiring treatment is related to the level of maternal stimulated TRAb levels, medical treatment of maternal disease. It crosses the placenta and stimulates the fetal thyroid.
Etiology of hyperthyroidism in pregnancy:28
Complications:28
Diagnosis of hyperthyroidism:28
Clinical signs and symptoms-
Laboratory assessment of serum TSH and fT3 & fT4 will show suppressed serum TSH with elevated fT3 & fT4 is diagnostic.
Measurement of antibodies
Antithyroid antibodies are common in patients with autoimmune thyroid disease, as a response to thyroid antigens. The two most common antithyroid antibodies are thyroglobulin and thyroid peroxidase (anti-TPO). Anti-TPO antibodies are associated with postpartum thyroiditis and fetal and neonatalhyperthyroidism.64 TSH-receptor antibodies include thyroid-stimulating immunoglobulin (TSI) and TSH- receptor antibody. TSI is associated with Graves’ disease. TSH-receptor antibody is associated with fetal goiter, congenital hypothyroidism and chronic thyroiditis without goiter. Recent studies investigated the relationship between the presence of antithyroid antibodies and pregnancy complications, finding a high proportion of women with previous history of obstetric complications and high levels of circulating anti-thyroid peroxidase antibodies and anti-thyroglobulin antibodies. Furthermore, thyroid function disorders may affect the course of pregnancy. Antibody patterns generally fluctuate with pregnancy, reflecting the clinical progress of the disease, but may remain stable in patients with low antibody titers. TRAbs can be detected in the first trimester, but values often decrease during the second and third trimesters and might become undetectable before increasing again postpartum. Clinically, patients can experience relapse or worsening of Graves' disease by 10-15 weeks of gestation. Graves' disease can, however, remit late in the second and third trimesters.65
Antibodies should be measured in the following conditions:66
Management
Thionamides like prophylthiouracil(PTU), methimazole (MMI) can be used. These drugs act by inhibiting the iodination of thyroglobulin and preventing thyroglobulin synthesis by competing with iodine for enzyme peroxidase (Figure 8).
Dose
PTU-100-150mg 8thhrly
MMI-10-20mg daily
Dose should be adjusted so as to maintain the acceptable level of TSH < 0. After achieveing euthyroid state, the dosage of PTU should be tapered to minimize fetal exposure to thionamides. If PTU & MMI are contraindicated β- blockers may be used to control adrenergic symptoms of thyrotoxicosis particularly tachycardia. In addition β-blockers block the peripheral conversion of T4 to T3. Propanalol 20-40 mg 2-3times a day is commonly used. Surgery must be reserved for the most severe cases. Radioactive iodine is an absolute contraindication in pregnancy. It is also important to continue the medication throughout postpartum period, as excerabation of graves disease is common then. Both PTU & MMI are compatible with breastfeeding.66 Antithyroid drugs are the treatment of choice for hyperthyroidism during pregnancy.67 They inhibit thyroid hormone synthesis by reducing iodine organification and coupling of MIT and DIT. Methimazole (MMI), propylthiouracil (PTU), and carbimazole have been used for the treatment of hyperthyroidism during pregnancy. The pharmacokinetics of MMI is not altered in pregnancy; it has been reported that serum PTU concentrations may be lower in the third than in the first and second trimesters of gestation. Use of PTU should be restricted to first trimester of pregnancy, after which change to MMI is recommended. Although adrenergic β- blocking agents may be used for the management of hyper-metabolic symptoms, their use should be limited to a few weeks because of possible intrauterine growth retardation and, if used in late pregnancy, they may be associated with transient neonatal hypoglycemia, apnea, and bradycardia.68
All antithyroid drugs cross the placenta and may potentially affect fetal thyroid function. Although PTU is more extensively bound to serum albumin than MMI and hypothetically less of it might be transferred through placenta than MMI, it has been shown that placental passage of PTU and MMI is similar. A study showed that transfer rates across the placenta were independent of the perfusate protein concentration, and this might be due to highly efficient placental extraction of the unbound drug. Cord PTU levels were higher than maternal concentrations in hyperthyroid pregnant patients treated with PTU until term. In addition, there were no differences in thyroid hormone and TSH concentrations in cord blood at birth between the MMI- and the PTU-treated newborn.68 Agranulocytosis was seen in 0.35–0.4% of patients using both antithyroid drugs. Vasculitis was seen more commonly with PTU and anti neutrophil cytoplasmic antibody positivity was 40 times more frequent with PTU than with MMI. Immunoallergic hepatitis occurs only with PTU, its frequency ranging between 0.1and 0.2%. PTU- liver failure is seen in one in every 10,000 adults and one in related 2,000 children and on average; it occurs 3 months after initiation of PTU therapy, although this complication may occur at any time during PTU treatment. In severe cases, up to 25–50% fatality has been reported and liver transplantation may be required. Therefore, it has been advised that PTU should not be prescribed as the first line agent in children or adults. However, due to the probability of association of fetal teratogenicity with MMI, PTU is still recommended as the drug of choice during the first trimester of pregnancy. Only two cases of liver failure have so far been reported with PTU in pregnancy.68
Three types of side effects of antithyroid drugs should be considered. A. Teratogenicity-There are two distinct teratogenicity patterns, aplasia cutis and choanal/esophageal atresia, reported with MMI use during pregnancy, but the data are controversial. Although multiple case reports of animal studies have been published associating aplasia cutis with MMI therapy in pregnant mothers , no case of aplasia cutis was seen in a series of 243 pregnant women treated with MMI , and the occurrence of aplasia cutis with MMI did not exceed baseline rate of 1in 30,000 births in normal pregnancies .Choanal and esophageal atresia may have a higher incidence than that expected in fetuses exposed to MMI during the first trimester of gestation or may be as high as18. However, the mother’s disease might be the causal factor rather than MMI treatment. A prospective cohort study did not show any significant difference in incidence of major anomalies or spontaneous abortions between MMI treatment and controls during pregnancy.68
Effects on the fetal thyroid
There is a lack of correlation between fetal thyroid function and maternal dosage of antithyroid drugs. Decreased serum-FT4 in 36% of neonates is seen when the maternal serum-FT4 is in the lower two-thirds of the normal non-pregnant reference range. Maternal thyroid status is the most reliable marker, and in pregnant mothers with serum-FT4 levels in upper third of the normal range, serum-FT4 concentrations of over 90%of their neonates are within normal range. Overtreatment of pregnant ladies with antithyroid drugs resulting in decreasing maternal serum-FT4 is usually accompanied by fetal hypothyroidism.68 Effect on pediatric physical and mental growth-No differences in thyroid function or physical and psychomotor development has been found between children born to MMI- or PTU-treated hyperthyroid mothers during pregnancy and those born to euthyroid mothers.68 Methimazole in doses of 10–20 mg or PTU 100–200 mg daily should be started, and after 1month, it is desirable to adjust the doses in order to maintain maternal fT4 in the upper one-third of each trimester-specific reference interval.68 FT4 and TSH should be monitored at monthly intervals during pregnancy. Serum TSH levels of 0.1–2.0 mU/l to be maintained. Surgery in pregnancy carries more risks than medical therapy and is complicated by hyperthyroidism. It is associated with an increased risk of spontaneous abortion or premature delivery.68 Thyroidectomy in maternal hyperthyroidism is rarely indicated, and subtotal thyroidectomy is indicated in patients with major or severe adverse reactions to antithyroid drugs, and if hyperthyroidism is uncontrolled because of lack of compliance, high doses of antithyroid drugs are required to control the disease and large goiter that may require high doses of antithyroid drugs (ATD).
The optimal timing for surgery is in the second trimester when organogenesis is complete, the uterus is relatively resistant to stimulating events, and the rate of spontaneous miscarriage is reduced. Some clinicians recommend discontinuation of antithyroid medications in the third trimester in 20–30% of pregnant women who have been euthyroid for several weeks on small doses and have low TRAb titers. A study has shown more recurrence of hyperthyroidism in the post partum period in those who had stopped antithyroid therapy compared with those who had continued such treatment throughout pregnancy and post partum.68 Neonatal hyperthyroidism is due to transplacental transfer of maternal TRAb and occurs in 5% of neonates of mothers with Graves’ disease.68 fT4 and TSH should be measured in the cord blood of any infant delivered by women with a history of Graves’ disease. If the woman was treated with ATD up to the end of pregnancy, clinical manifestations of neonatal hyperthyroidism may be only seen for the first time a few days after delivery, because the fetus was protected by the ATD received from the mother during the final weeks of gestation. Antithyroid treatment and propranolol should be initiated. Either MMI 0.5–1 mg/kg or PTU 5–10 mg/kg daily should be given to neonates with hyperthyroidism. Propranolol 2mg/kg daily is helpful to slow down pulse rate and reduce hyperactivity in ill neonates. Lugol solution or potassium iodide and glucocorticoids may also be given in more severe cases.68
Cases of thyrotoxicosis due to Graves’ disease occur more frequently during the post partum period than at other times in women of childbearing age .In the months following delivery, exacerbation of immune reactivity occurs between 3 and 12 months post partum. Therefore, autoimmune thyroid disorders may begin to recur or exacerbate during this crucial period. Graves’ disease and post partum thyroiditis are two major causes of thyrotoxicosis in the first year after delivery. Thyrotoxicosis caused by post partum thyroiditis usually does not require treatment; therefore, it is of utmost importance to differentiate between Graves’disease and post partum thyroiditis, TSH receptor antibodies being positive in the former and negative in the latter.68 If a woman is not breastfeeding, radioiodine uptake may show low values in postpartum thyroiditis and elevated or normal values in Graves’ disease. Antithyroid drugs are the mainstay of treatment for thyrotoxicosis during post partum period. Neither PTU nor MMI causes any alterations in thyroid function and physical and mental development of infants breast-fed by lactating thyrotoxic mothers. Methimazole is the preferred drug, because of a risk of potential hepatotoxicity of PTU in either mother or child.68
Complications of methimazole include:
Thyroid autoimmune disease in pregnancy & after delivery
Graves’ disease
In females in the reproductive period, GD is the most frequent cause of hyperthyroidism, which occurs in the population with an estimated prevalence of approximately 1%44. Among pregnant women the prevalence rate of overt hyperthyroidism is approximately 0.1–0.4% and GD accounts for 85–90% of all cases.69 In this type of thyroid autoimmunity, the humoral immune response predominates with the characteristic appearance of stimulating antibodies against TSH receptor (TRAbs), causing hyperthyroidism, goiter and nonthyroid manifestations, such as Graves’ orbitopathy or dermopathy.
Owing to physiological immunosuppression during pregnancy the development of GD or relapse of hyperthyroidism in this period is rare, usually emerging in the first trimester of pregnancy. In the second half of pregnancy even the gradual improvement of previously existing hyperthyroidism is frequently observed, being most probably the reflection of the stimulating TRAbs decrease in the second and third trimester.70 In the postpartum period, when the immunosuppression ceases, the increase of stimulating TRAbs,64 together with relapse of GD, is frequently observed, usually between 4 and 8 months after delivery. In the recent study of patients in remission after antithyroid drug treatment, the recurrence of GD was determined in 84% of patients in the postpartum period compared with only 56% of patients not being pregnant.71 However, as indicated by one single study, the postpartum period itself has not been shown to be a major risk factor for the first onset of GD.72 Untreated or inadequately treated GD in pregnancy may lead to several detrimental complications. In mothers, hyperthyroidism has been associated with preeclampsia and with the increased risk of congestive heart failure and thyroid storm. In the pregnancy course, hyperthyroidism may increase the risk of miscarriage, stillbirth, preterm delivery and placental abruption. Fetal hyperthyroidism, which occurs in less than 0.01% of pregnancies,73 may lead to tachycardia, fetal goiter, accelerated bone maturation, growth retardation, low birth weight and malformations. In the fetus, the excess of thyroid hormones may be the reflection of the mother’s thyroid hormones or the mother’s stimulating TRAbs crossing the placenta. Those antibodies have the impact on fetus only after the twelfth week of gestation, when the fetal thyroid starts to respond to the stimulation.53 In late pregnancy they represent a risk of neonatal hyperthyroidism, which occurs in up to 5% of newborns of mothers with GD. It usually persists for up to 12 weeks due to slow clearance of maternal antibodies, having a half-life of approximately 3 weeks.70
Hashimoto’s thyroiditis
With the estimated prevalence of 18% in the population, HT is probably one of the most prevalent autoimmune disorders in general. In women in the reproductive period, the prevalence of thyroid antibodies was approximately 10–15% and the prevalence was increasing with age.44 In contrast to GD, in HT the cell-mediated immune response predominates with consequent gradual destruction of thyroid tissue, which frequently leads to hypothyroidism. In pregnancy, the TPOAb and TgAb were shown to decline gradually with the lowest values in the third trimester, while the increase was observed as soon as 6 weeks after delivery and returning to the pre-pregnant values 12 weeks after delivery.40 In HT, both hypothyroidism and thyroid autoantibodies have been implicated to be involved in pregnancy complications. Overt or subclinical hypothyroidism, occurring in approximately 2–4% of apparently healthy women, has been related to two–threefold increased risk of gestational hypertension, placental abruption, postpartum hemorrhage, preterm delivery or miscarriage. Besides increased risk of low birth weight, neonatal respiratory distress and fetal abnormalities, such as hydrocephalus and hypospadias, maternal hypothyroidism during pregnancy has also been demonstrated to affect neuropsychological development of the child.20 However; rapid and adequate correction of hypothyroidism with l-thyroxine therapy has been shown to improve obstetrical outcome.20. In euthyroid pregnant women, elevated thyroid autoantibodies have been associated with two-to four-fold increased risk of miscarriage and with up to threefold increased risk of preterm delivery, although the etiology remains unresolved. Those complications may be associated with underlying generalized immune imbalance, with subtle deficiency of thyroid hormones due to thyroid autoimmunity, or with older age of those females.20 Hypothyroidism may also lead to infertility, since menstrual irregularities, including oligomenorrhea, menorrhagia and ovulatory dysfunction may occur and their severity correlates with the elevation of serum TSH levels. Similarly, hypothyroidism may provoke in vitro fertilization failure in infertile females, while l- thyroxine replacement has been shown to improve embryo implantation rate and pregnancy outcome. However, the clinical importance of thyroid antibodies in infertility remains controversial and underlying pathogenic mechanisms of putative association still need to be clarified.20
Postpartum thyroiditis
Postpartum thyroiditis refers to thyroid dysfunction within the first year after delivery or miscarriage, when the known immunosuppressive effect of pregnancy disappears. The clinical disease may present with hyperthyroidism alone, only with hypothyroidism, or with hyperthyroidism followed by hypothyroidism. The prevalence varies significantly between studies from 1.1 to 21.1% 7, with estimated pooled prevalence in the general population of approximately 8%, occurring up to six-times more often in females with elevated TPOAb and three-times more often in females with Type 1 diabetes 74. Therefore, in these two groups screening for thyroid dysfunction is recommended 3 and 6 months after delivery.20 Females positive for TPOAb in early pregnancy develop PPT in 40–60% of cases, while among patients with PPT 70% present with positive TPOAb, putting them at risk for developing a permanent thyroid dysfunction.40 The hyperthyroid phase of the disease is only transient, more frequently occurring in TPOAb-negative patients between 1 and 6 months after delivery and lasting 1–2 months. Hypothyroidism may occur with or without a previous hyperthyroid phase, more often in TPOAb-positive patients and between 3 and 8 months after delivery, being caused by destruction of thyroid tissue 407. It may be only transient, lasting 4–6 months and passing within 1 year after delivery or it may be permanent.20 A few earlier studies reported permanent hypothyroidism in up to 30% of PPT patient,20 but a recent large prospective report demonstrated a significantly higher incidence of approximately 50%. The latter observation might be an overestimation, since owing to limited sampling only 6 and 12 months after delivery a considerable number of patients with transient hypothyroidism may have been missed32–75 However, patients with transient hypothyroidism are also at risk for developing permanent hypothyroidism, which is established within 5–10 years after PPT in 20–60% of females.74 While in the hyperthyroid phase no specific antithyroid therapy is indicated, replacement therapy with l-thyroxine frequently needs to be started in hypothyroid patients.20
Pregnancy-specific conditions that lead to hyperthyroidism
There are two pregnancy specific conditions, hyperemesis gravidarum and trophoblastic disease, that can lead to hyperthyroidism. These conditions need to be identified as soon as possible because treatment of the underlying disease will resolve the hyperthyroidism.
Hyperemesis gravidarum
The syndrome of transient hyperthyroidism of hyperemesis gravidarum should be considered in any woman presenting in early pregnancy with weight loss, tachycardia, and vomiting and manifesting biochemical evidence of hyperthyroidism. Hyperemesis gravidarum is characterized by severe vomiting, which begins at; 6–9 weeks of gestation and usually resolves spontaneously by 18–20 weeks. This disorder occurs in; 0.2% of pregnancies.28 Of patients with hyperemesis gravidarum, as many as 60% exhibit hyperthyroidism.76 They have no history of thyroid illness preceeding pregnancy, goiter is usually absent, and thyroid antibodies are negative. On laboratory examination, the serum free T4 is more frequently increased compared with the serum free T3 concentration. In addition, when hyperthyroidism is present, patients are more likely to have abnormal electrolytes and liver function tests. Interestingly, more severe vomiting is associated with a greater degree of thyroid stimulation and higher concentration of hCG.77 The etiology of transient hyperthyroidism of hyperemesis gravidarum is unclear. Some have argued that the hyperthyroidism is the cause of the hyperemesis, whereas others have argued the reverse.28 A recent report,29 describing two patients with hyperemesis and hyperthyroidism attributable to hCG-hypersensitive thyrotropin receptors, suggests that hyperemesis can be directly related to the overactive thyroid and not necessarily to the effects of excess hCG. Treatment for transient hyperthyroidism of hyperemesis gravidarum involves rest, a controlled diet, and antiemetic therapy. The hyperthyroidism generally resolves with the cessation of vomiting. Gestational trophoblastic disease. Hyperthyroidism can also occur in women with gestational trophoblastic disease (GTD). GTD is a general term that includes benign and malignant conditions of hydatidiform mole (both complete and partial) as well as choriocarcinomas. The frequency of hydatidiform mole is approximately 1 in 1,500–2,000 pregnancies and that of choriocarcinoma is 1 in 40–60,000.28 The frequency of hyperthyroidism in GTD has been estimated as anywhere from 5% to 64%.27 The etiology of the hyperthyroidism is thought to be related to the increased concentrations of serum hCG in these patients, which can be as high as 1,000-fold higher than reference values28. As mentioned previously, prolonged increases in serum hCG can clearly cause a significant increase in hyroidfunction.20 Hyperthyroidism attributable to GTD should be suspected in patients who demonstrate increased free T4 and T3 concentrations, decreased TSH, and significantly increased hCG. The thyroid gland is either not enlarged or only slightly enlarged, rarely to more than twice normal size, and ophthalmopathy is absent. Complete surgical removal of the GTD rapidly cures the hyperthyroidism.
The physiology of fetal thyroid
The fetal hypothalamic-pituitary-thyroidal system develops and functions autonomously. The transplacental passage of T4 and T3 is minimal both in animals and man.78 There is no correlation between maternal and fetal concentrations of T4, T3 or TSH at any time during gestation despite a concentration gradient. Furthermore, only minimal proportions of T4, or radioiodine-labelledT4, given to mothers before labour or therapeutic abortion have been detected in the fetus. Animal studies support this conclusion, but biologically active thyroid hormone analogues may cross the placenta in some species.78 The fetal thyroid does not secrete thyroid hormone until the end of the first trimester and its development proceeds in the absence of TSH. There is an abrupt rise in fetal TSH concentrations between 18and 24 weeks correlated temporarily with histological maturation of the hypothalmic-pituitary-portal vascular system, which results in a marked increase in thyroidal production of T4 and T3. The concentration of T4, especially free T4 (as fetal TBG concentration does not increase), rises slowly after 30 weeks to that of the mother at term, whereas the elevated fetal TSH levels decline somewhat towards term.78 The peripheral de-iodination of T4 is the major source of production of T3 andalmost exclusively the source of reverse T3. In the fetus, the peripheral de-iodi nation of thyroxine favours the production of biologically inactive reverse T3 at the expense of active T3; thus cord blood T3 concentration is approximately one fifth that of Maternal79 During the neonatal period there is a marked increase in serum TSH, in part a response to neonatal cooling, reaching a peak at 30 min. Serum T3 also increases, reaching an early peak at 2 hr and a second peak coincidental with the T4 and free T4 peak at 24 hr. This period of neonatal thyroid hyperactivity is transient, falling gradually over 2 to 3 days for TSH and 2 to 3 weeks for the thyroid hormones. Reverse T3 levels do not peak and return to adult range within 10 to 14 days suggesting maturation of the peripheral enzymatic pathway of thyroxine metabolism. It is now possible to recommend screening of the newborn for hypo thyroidism, based on T4 or TSH, or preferably both, on either cord blood orheel prick 3 to 5 days post partum. The incidence of neonatal hypothyroidism varies from 1/4000 in a number of European countries to 1/7000 in Quebec.80 It is during the first trimester of pregnancy that the thyroid hormones are most important to fetal brain development. Still significant fetal brain development continues considerably beyond the first trimester, making the thyroid hormones important also later in gestation. Overt maternal thyroid failure during first half of pregnancy has been associated with several pregnancy complications and intellectual impairment in the offspring. It is currently less clear whether milder forms of thyroid have similar effects on pregnancy and infant outcome.
Comparasion of different studies
Abortion |
2.10% |
Dr Tanuja PM, et al. (1.7%) |
Anaemia |
4.20% |
Dr pavanaganga et al. (5.08%) |
Ajmani Sangita, et al. (14.1%) |
||
PIH |
14.70% |
Leung (15%) |
Hulya Ozdemir (14.5%) |
||
GDM |
4.20% |
Dr Pavaganga et al. (6.4%) |
Das Bishnu Prasad et al. (8%) |
||
Pre term labour |
3.10% |
Sahu MT (4.7%) |
Ajmani Sangita et al. (11.2%) |
||
Oligohydromnios |
16.70% |
Dr Pavanaganga et al. (8.3%) |
LSCS |
22.90% |
Mary George1 et al. (20%) |
Ajmani Sangita et al. (16.6%) |
||
PPH |
6.30% |
Mohammed M et al. (6%) |
Ajmani Sangita et al. (5.5%) |
||
LBW |
21.90% |
Hareesh MV et al. (16.32%) Ajmani Sangita (25%) |
Hyperbilirubinemia |
9.40% |
Mary George1 (8%) |
NICU ADM |
14.60% |
Dhara Singh Meena (14.28%) |
Thyroid disease is common in women of reproductive age, and it is most common endocrine disorder after diabetes. Subclinical hypothyroidism is the most common. Maternal thyroid deficiency even subclinical has been associated with adverse pregnancy outcome and may be improved by T4 replacement.81 Fluctuations occur in T4 metabolism during pregnancy make it difficult to maintain meticulous normal thyroid harmone values during gestation in hypothyroid mothers.82 Pregnancy cause increased thyroid gland vascularity, increased renal iodide clearance and iodide losses to the fetus.83 Fluctuation in thyroxine metabolism that occur during pregnancy may further impair maternal-fetal transfer of thyroxine despite apparently optimal thyroid status.84 About 10% of pregnant women who have TPO antibodies during early gestation are at increased risk of developing subclinical hypothyroidism during pregnancy and thyroid dysfunction post partum. The latter condition occurs in 5-9% of women and 25-30% progress to permanent hypothyroidism (1.7%). Presence of antibodies to thyroid peroxiadase (TPO-AB) or thyroglobulin during pregnancy is associated with significant increment in miscarriages, premature deliveries, geastational diabetes, postpartum thyroiditis and permanent hypothyroidism85–88 Dr Pavanaganga et al.,85 it is 6.4% .The increase in preeclampsia incidence, of the pre mature delivery, of the post partum depression and haemorrhages could be explained through a maturation process of the placenta and they appear especially if there is a severe Hypothyroidism, but they have been also signaled in the cases of subclinical hypothyroidism.
Anaemia in subclinical hypothyroid patients is seen in 5.08%. Hemotopoietic system is the primary affected systems resulting in anemia. thyroid hormones play a role in hematopoiesis. The most frequent reason of this is the bone marrow supression due to thyroid hormone deficiency as well as lack of erythropoietin production.
Oligohydromnios in subclinical hypothyroid patients is in a study done by dr avanaganga et, it is 8.35%. Pre term labour in subclinical hypothyroid patients in a study done by Sahu M T is 4.75%. PPH in subclinical hypothyroid patients in a study done by ohammed et al., is 6% subclinical hypothyroid patients with a study done by is (20%). The utero placental interface is susceptible to both thrombosis and haemorrhge, particularly in association with structurally defective placentation. Several factors may mediate such pathogenesis: for ex tissue factor production in response to aberrant vascular ndothelial growth factor and inflammatory cytokine release, which promotes thrombosis. In addition, shallow extra villous thromboblast invasion (EVT) may lead to placental ischemia and haemorrhage generating thrombin locally, which mediates the degradation of extracellular matrix, thus triggering pre mature placental separation from the uterus.89 In a study by, abruption placenta rate is 0.3% , where as in our study we did not come across any patient with abruption.
Reduced foetal thyroxine may cause disruption to the development of the pituitary-thyroid axis of the newborn, fetal pituitary GH secretion, vascular responsiveness and maturation, cardiovascular homeostasis in utero.90–92 These factors may be responsible for observation of reduced neonatal birth weight of offsprings born to mothers with inadequately controlled thyroid at initial presentation or at 3rd trimester. LBW in subclinical hypothyroid patients with a study conducted by Ajmani Sangeeta, et al is (25%) Most of the low birth weight babies were due to PIH in mother, leading to IUGR and low birth weight. Hyperbilirubinemia in subclinical hypothyroidism is 9.4% which is co-relating with a study done by Mary Georgel et is (8%) NICU admission in subclinical hypothyroid patients is with a study done by Dhara singh Meena et (14.28%). Oral pathology was not so significant among pregnant patients with thyroid disorder, although delayed tooth eruption was seen in 12.6% of infants with mothers with hypothyroidism. Infants born to hypothyroid women are at increased risk of impairement in neuropsychological development and IQ scores. All women with overt and subclinical hypothyroidism should be treated irrespective of TPO antibody positivity with LT4 during pregnancy to maintain serum TSH in the trimester specific goal range. It has been recommended to check the serum TSH level every 4 weeks during pregnancy so that appropriate dose adjustments can be made. The recommended therapy is oral LT4 which should be given on empty stomach (45 min before consumption of food, beverages, or other medications). In addition, calcium, iron, and prenatal vitamins supplement should be avoided within 4 hours of ingestion of LT4, as these can decrease the absorption of thyroxine. In a typical case dose requirement goes up as pregnancy advances as pregnancy is a hypermetabolic condition.
Immediately after delivery, the requirement of thyroxine drops and women who were taking thyroxine prior to pregnancy will shift to their pre pregnancy dose and those who started thyroxine in pregnancy will require half the dose they were taking just before the delivery. In women who had started thyroxine in pregnancy for subclinical hypothyroidism, the medication can be stopped after delivery and thyroid balance re assessed again after 6 weeks and decision taken regarding continuation of treatment. Serum T3, T4 levels rise 30 minutes after delivery and persist for 5 days. This is due to TSH elevation caused be the stress of delivery. So new born screening should be done from cord blood or 5 days after delivery.
Thyroid testing is a must at first booking, ideally pre natally to prevent miscarriages. As fetus needs thyroxine for brain development, growth, and lung maturation, adequate replacement therapy should be done to keep TSH with in trimester specific referrence ranges. Early and effective treatment of thyroid disorders ensures safe pregnancy with minimal maternal and fetal complication.
None.
The author declares there is no conflict of interest.

©2018 Sreelatha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.