eISSN: 2473-0815


Review Article Volume 5 Issue 5
1Medical director of Pronokal Group, Spain
2Medical director of Pronokal Group, Carrer Roger de Ll, ria, 58, 08009 Barcelona, Spain
3Endocrinology and Nutrition Service of Hospital Clínic de Barcelona, IDIBAPS Translational Research Group on Diabetes, Lipids and Obesity (Institut d’Investigacions Biomèdiques August Pi i Sunyer), Spain
Correspondence: Ignacio Sajoux, Medical director of Pronokal Group, Carrer Roger de Ll, ria, 58, 08009 Barcelona, Spain, Tel +34 666988021
Received: August 28, 2017 | Published: October 24, 2017
Citation: Sajoux I, Bellon A, Vidal J. Challenges in treatment of obesity in the elderly. Endocrinol Metab Int J. 2017;5(5):291-297. DOI: 10.15406/emij.2017.05.00135
Obesity is a very common problem that affects one in three people over 65 in Spain. In addition to the risk derived from the appearance of comorbidities in organs and systems, obesity in seniors exhibits a particular set of characteristics, such as loss of muscle mass and a specific distribution of fat which directly contributes to an increase in morbimortality. The purpose of this review is to study the epidemiology and physiopathological mechanisms of obesity in the elderly, as well as the therapeutic approach aimed at preserving lean mass in these patients. An extensive bibliographical search was carried out using scientific publications in various specialised electronic databases (PubMed, Scielo and Elsevier) and Google Scholar. For obese patients over 65 years of age, preserving muscle mass is a priority in any weight-loss programme, as this is associated with decreased cardiometabolic risk and increased resistance which in turn inversely correlates with mortality. For this reason, reducing calorie intake while maintaining a high protein intake is recommended. This goal can be attained through a very-low-calorie diet accompanied by a personalised physical exercise programme to improve the patient's functional capacity.
Keywords: obesity, sarcopenia, elderly, diet, weight loss
Obesity is a multifactorial disease that appears as a result of the interaction between the genotype and the environment. In developed countries it affects a high percentage of the population of both sexes of all ages and social economic levels. Its prevalence has been increasing alarmingly in recent decades1 and it has continued rising to reach epidemic proportions.2
In Spain, the highest prevalence of obesity occurs in people over 65 years-old, predominantly abdominal obesity, strongly associated with the occurrence of insulin resistance and an increase in cardiovascular risk.3,4 In addition, the elderly have a number of special circumstances that contribute to increasing morbidity and mortality, such as loss of muscle mass and a redistribution of body fat, which tends to decrease in the subcutaneous tissue and accumulate in the muscle and in the intra-abdominal zone. These changes facilitate the development of metabolic alterations (Type 2 Diabetes, Dyslipidemia, etc.) and condition functional and fragility issues (falls, fractures, degree of dependence).5
The purpose of this article is to carry out a descriptive review of obesity in patients over 65 years of age. We cover epidemiological aspects, the peculiarities of pathophysiological mechanisms in this age group and the available therapeutic options.
Diagnosis and classification of obesity in the elderly
Obesity is characterised by an excess of body fat. According to quantity, we can define obese individuals as those whose percentage of body fat is greater than 12%-20% in adult men and 20%-30% in adult women, which are considered normal values.6
In young and middle-aged people, the Body Mass Index (BMI) is a reliable and simple correlate for estimating body fat. In addition, there are numerous epidemiological data that associate BMI with the health risk of a deficit in or excess body weight. However, in the elderly this relationship is less evident due to the changes in body composition that occur at this stage of life, specifically an increase in the amount of intramuscular fat and its accumulation in the abdominal region. Thus, although the BMI cut-off points classifying obesity do not vary according to age, their translation in terms of excess of body fat - that which actually defines obesity - is not the same for young people and those aged older than 65.7
When there is also an excess of intra-abdominal adipose tissue, as occurs in elderly patients, morbidity increases. In these cases, the abdominal perimeter, the waist-to-hip ratio or waist-height ratio are better indicators of cardiovascular risk than BMI.8,9
Prevalence of obesity in the elderly population in Spain
During the last 20 years, obesity has been increasing.3 Most of the epidemiological studies carried out in Spain have focused on the study of obesity in children and in middle-aged adults, but some studies, such as the ENRICA study (Nutrition and Cardiovascular Risk Study in Spain), also evaluated obesity in the elderly. According to the results of this study carried out on 12,883 representative individuals of the non-institutionalised Spanish population, 22.9% of Spaniards are obese (24.4% of Men and 21.4% of Women) and have a BMI equal to or greater than 30 kg/m2.
Frequency increases with age, affecting 35% of the population who are over 65 years-old (30.6% in men and 38.3% in women). Abdominal obesity, understood as a waist circumference greater than 102 cm in men and 88 cm in women, was observed in 36% of the population studied (32% in men and 39% in women) and in 62% of those over 65 (51.4% in men and 70.7% in women).
Comorbidities and mortality in obese people aged over 65
Obesity is associated with significant comorbidities that significantly deteriorate quality of life and expectancy. In the elderly, these complications manifest mainly in metabolic syndromes, including glucose intolerance, hypertension, dyslipidemia and cardiovascular disease.10 High blood pressure is the most constant comorbidity in the obese, especially in the elderly and notably when abdominal obesity is present.11,12 In a study of one million people, the prevalence of high blood pressure in those who were overweight was 50%-300% higher than in those with normal or low body weight.13 In the Framingham study, prevalence also increased with higher body weights14 and it was estimated that 70% of new cases of hypertension could be attributed to obesity or weight gain.15
Other common complications of obesity in older adults are type 2 diabetes mellitus, osteoarthritis, dyslipidemia, sleep apnea, coronary and cerebrovascular disease, cholelithiasis, heart failure, and some cancers such as breast and endometrium in women and colon, prostate and rectum in men.6 Likewise, these patients often present functional limitations due to the decrease in muscle mass and strength, in addition to joint problems, disabilities in daily activities, fragility, chronic pain and deterioration of quality of life.
Obesity reduces survival rates in the middle-aged and elderly groups. The risk of dying is two to three times higher for obese people than those whose BMI is between 23.5 and 24.9, mainly because of cardiovascular disorders, but also due to cancers related to obesity, diabetes and renal disease.16 When the obese person is elderly, the disease is accompanied by a loss of muscle mass, whose decrease is also correlated with impairment of functionality and an increase in mortality from any cause.17-20
Survival rates show a bimodal curve where mortality increases with both high and very low BMIs. In an epidemiological study conducted in the United States on a cohort of 527,265 individuals of both sexes aged 50-71 at the time of inclusion, it was found that after 10 years of follow-up, mortality was higher in those who had the highest and lowest BMIs.21 Other studies also show that the relative risk of dying is higher in those individuals whose BMI is greater than 30 or lower than 18.5 kg/m2, with the highest differences being in those older people aged over 60.22 A recent meta-analysis on the risk of mortality from any cause associated with BMI has shown that, in the population aged over 65, the presence of over weightness is associated with lower mortality.23 This phenomenon is due in part to the fact that many elderly people have inadequate nutrition, and a reduction in BMI can also imply a greater loss of muscle mass, whose negative effects could counteract the benefit of having a fat mass percentage that is within normal limits.
Characteristics and pathophysiological mechanisms of obesity in the elderly
Changes in body composition occur with the aging process that are characterised by a progressive increase in fat mass and a decrease in muscle or lean mass. The amount of fat mass increases progressively until 80 years of age and then stabilises, while muscle mass tends to decrease from 40-50 years.24,25
The observed changes in fat mass that occur over the years include a decrease in the amount of subcutaneous fat and an increase in visceral and intramuscular fat. The decrease in the amount of subcutaneous fat is due to a progressive decrease in the capacity of the subcutaneous adipose tissue to store lipids, especially in the lower limbs. In addition, there is a loss of muscle fibres and of the amount of muscle mass (more marked in the lower limbs), the mitochondrial function is impaired, telomeres are shortened and the expression of inflammatory cytokines increases.26 The smaller amount and size of muscle fibres translates into a loss of muscle strength and functional capacity, which deteriorate the quality of life, increase the risk of disability, morbidity and mortality and increase the risk of fragility, falls and dependence.27
The term sarcopenia was coined by Rosenberg in 1989 to describe the decline in skeletal muscle mass and strength as a result of ageing.28,29 According to EWGSOP (The European Working Group on Sarcopenia in Older People), the diagnostic criteria for sarcopenia are loss of muscle mass, decrease in muscle strength and decrease in physical performance; the first and some of the other two criteria must be met in order to confirm the diagnosis.30 According to data from cross-sectional studies, muscle strengths peaks between the second and third decades of life and remains stable until 40-50 years. Thereafter, it begins to decline at a rate of 1%-2% per year between the ages of 50 and 60 and following that decade at a rate of 3% per year.31
Changes in body composition in older adults often lead to a combination of being overweight and a reduction in lean mass (including muscle and bone mass). This has given rise to the concept of sarcopenic obesity,32 which is characterised by the presence of insulin resistance and chronic inflammation and raising the degree of disability and mortality in patients, due to the boosting effect of both combined.5,33,34
Skeletal muscle is the tissue predominantly responsible for the consumption of glucose mediated by insulin; because of this, in situations of reduction of muscle mass and increase of adiposity, glucose tolerance decreases.35 Thus, loss of muscle mass associated with age can contribute to the development of insulin resistance (Figure 1), which in turn aggravates the loss of skeletal muscle, creating a vicious cycle. This process is even more aggravating in the elderly who also have increased visceral fat mass.36
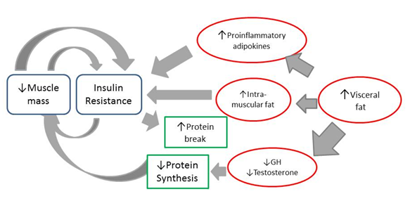
Figure 1 Metabolic pathway of insulin resistance associated with loss of muscle mass.
Footnote to Figure 1 The loss of muscle mass associated with age contributes to the development of insulin resistance which in turn aggravates the loss of skeletal muscle. In addition, an increase of vsiceral fat in the obese, elderly patient is associated with a production of proinflammatory adipokines that have a positive association with fat mass. Insulin resistance and systemic inflammation stimulate protein breakdown and suppression of muscle synthesis. Obese sarcopenic patients have greater insulin resistance than only obese or only sarcopenic patients.
Insulin regulates the amount of skeletal muscle tissue by inducing changes in protein degradation.37 Under normal conditions (Figure 2a) the binding of insulin to its receptor activates the phosphatidylinositol 3-kinase (PI3K) protein and the serine threonine kinase (AKT) protein. Activation of the AKT protein leads to a decrease in proteolytic activity mediated by the suppression of caspase-3 activity and FoxO (Forkhead box O) protein. In insulin-resistance states (Figure 2b) PI3-kinase and AKT activity is reduced, resulting in an increase of proteolytic activity and a reduction in lean mass.37
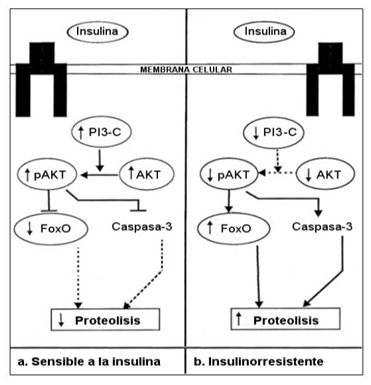
Figure 2 Control of lean muscle mass in the insulin sensitive and insulin resistance states.
Footnote to Figure 2 a) normal insulin sensitivity; b) insulin-resistant. Adapted from Honors MA et al.37
For the obese, elderly with loss of muscle mass, this creates a vicious cycle that will lead to a greater degree of sarcopenia and deterioration of their metabolic and inflammatory state. These patients have a greater degree of insulin resistance than those who are only obese or only sarcopenic.38 Weight loss is associated with loss of lean mass, which can aggravate their sarcopenia. Therefore, minimising lean mass loss is a valid objective for interventions whose purpose is to achieve weight loss in patients aged over 65.39
Screening for sarcopenic obesity
Sarcopenic obesity has a significant clinical impact on patients. A decrease in lean or muscular mass reduces strength and muscle tone, resulting in an increase in the risk of falls which, because of the loss of bone tissue (osteopenia) in sarcopenic patients,40 can lead to fractures that produce disability and dependence (Figure 3).
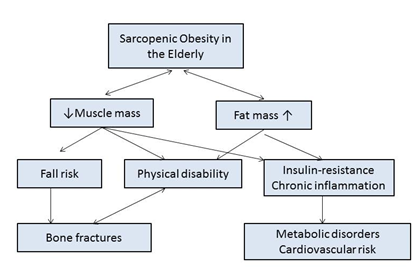
Figure 3 Clinical impact of sarcopenic obesity in the elderly.
Footnote to Figure 3 Adapted from Kim TN et al.38
When fat mass also increases, insulin resistance occurs, which, when associated with patients’ present chronic inflammatory state, increases their cardiometabolic risk.41 Increasing obese, elderly and sarcopenic patients’ amount of lean mass is a priority in weight reduction programmes, as it translates into an improvement in insulin sensitivity that will help reduce their cardiometabolic risk.
In the obese, elderly and sarcopenic patient, excess fat mass and a deficit in lean mass are mutually enhanced, maximising their detrimental effects on disability, morbidity and mortality.5,42 Therefore, identifying these people is necessary, even from a state of presarcopenia (loss of muscular mass with strength and still conserved physical performance), in order to initiate a treatment that prevents the progression of the disease and thus reduces its clinical impact.
Therapeutic approach to obesity in the elderly
Inducing weight loss in obese and elderly patients improves their functional status and quality of life and reduces associated medical complications. For this age group, in which the percentage of fat mass increases and the percentage of lean mass decreases at the expense of muscle mass, the American Society for Nutrition (ASN) and the North American Association for the Study of Obesity (NAASO) recommend choosing treatments that focus on weight losses while simultaneously minimising the loss of muscle tissue and bone tissue, whose deficiency leads to an increased risk of fractures.43
Interventions on life-styles
One of the most common therapeutic approaches is to encourage the patient to adopt a sustained change in their lifestyle. Standard hypocaloric diets are usually indicated, together with an exercise programme and a series of behavioural changes that are aimed at motivating patients and helping them comply with hygienic-dietary measures. However, although this therapeutic approach has certain logic for obese young and middle-aged patients, these measures might further reduce muscle mass in obese, elderly patients and exacerbate deficits in physical function.44 It is estimated that 75% of the weight reduction achieved through diet corresponds to fat tissue and 25% to lean tissue. This moderate loss of lean tissue is not considered important in young adults as, because of their excess weight they also have a greater muscle mass, but it is relevant in the case of older patients.
The combination of a low-calorie diet, increased physical activity, and changes in behaviour or lifestyles in patients aged over 60, results in moderate weight loss (0.4-0.9 kg per week or 8-10% of weight at six months), with an improvement of obesity-related comorbidities, improvement of physical dysfunction and a small risk of complications associated with treatment. These interventions achieve significant improvements in glucose tolerance and physical functioning, reducing the incidence of diabetes and result in significant benefits for people with osteoarthritis, diabetes and ischemic heart disease. However, they are also associated with certain negative results such as loss of lean body mass and bone mineral density.45
In elderly patients, the combination of diet and physical exercise obtains the best results, both in weight reduction and in decreasing mortality.46
Diet and supplementation
For the treatment of patients aged over 65 who are obese and losing lean mass or have sarcopenia, a diet that includes moderate caloric restriction (200-750 kcal, or 840-3,150 kj) and a higher intake of high biological quality protein is recommended; this will prevent loss of muscle mass and ensure adequate kidney function. The current recommendation is to administer elderly proteins with more proteins than those recommended for the general population, given it has been found that a daily intake of 1.2 to 1.5 g of protein per kg of body weight can better maintain lean mass and result in less loss of bone tissue, maintaining an adequate glomerular filtration rate in patients with normal or slightly deteriorated renal function.47-50
Maintaining or even increasing the supply of protein and amino acids through diet are the most effective ways to delay or prevent catabolism of muscle proteins. In addition, the muscles of elderly patients require a large amount of protein and amino acids to stimulate protein synthesis, to a similar degree to that of young patients.51 Dietary intervention based on caloric restriction and high protein content has been shown to provide benefits in the long-term survival of obese sarcopenic patients.52,53
Very low-calorie diets (VLCD) are a dietary treatment modality for obesity that helps preserve lean mass. They are diets, usually ketogenic, that prescribe less than 800 calories a day (between 450 and 800 kcal/day), although the caloric deficit that requires this measure will depend on the energetic requirements of each patient, so some describe the diets as one that prescribe less than 50% of the patient's energy expenditure or less than 12 kcal per kg of ideal weight.54
According to the report prepared by SCOOP [55], VLCD diets should contain high biological value protein (0.8-1.5 g per kg per day) and provide the recommended amounts of vitamins, minerals, electrolytes and essential fatty acids needed to obtain a significant weight reduction while providing adequate nutrition and preserving lean mass.55,56 These diets together with physical exercise do not reduce lean mass, as evidenced by the results of two recent studies carried out in young and middle-aged patients. In a controlled, two-year follow-up study into obese patients (mean BMI, 35.1 kg/m2), where those who were treated with a VLCD had lost an average of 22.0 kg at six months, 19.9 kg per year (p <0.0001) and continued to maintain a mean weight loss of 8.8 kg at two years, there was practically no change in lean mass during the 24 months of study,57,58 and it was found that the VLCD diet was safe, well tolerated and significantly more effective than the standard hypocaloric diet. In another study, in which body composition changes were assessed by dual energy densitometry (DXA), multifrequency bioelectrical impedance analysis and air displacement plethysmography in obese patients who lost weight by following a VLCD for four months, it was found that loss of weight (an average of 20.2 kg in four months) occurred mainly at the expense of body fat and visceral mass, while muscle mass and strength were maintained.56 In obese, elderly patients, VLCD should be used under medical supervision, especially when they have comorbidities, as these patients usually present a loss of lean mass and their vitamin and mineral requirements are often higher than young obese and middle-aged patients.55,59
Another strategy to improve protein synthesis is to administer essential amino acid supplements (arginine, glutamine and lysine) and increase leucine intake, as it promotes anabolism and decreases protein degradation.60 In addition, it has been observed that it stimulates muscle protein synthesis by 56%.61 Supplementation with hydroxymethylbutyrate (HMB), the active metabolite of leucine, is also effective in increasing total protein synthesis.62-64 Other specific micronutrients to be addressed in the obese elderly are vitamin D, involved in protein synthesis and muscle health,65 magnesium (whose deficit is associated with insulin resistance), vitamins B6 and B12 and selenium, whose deficit is associated with functional impairment.
Physical exercise
Physical exercise combined with the diet is essential to treat obese patients because it improves their functional status and contributes to maintaining muscle mass and bone tissue and improving muscular strength; the greater the loss of fat mass, the better the results.66 With exercise, intramuscular fat is reduced, improving the quality and functional capacity of the muscle and decreasing levels of TNFα and IL-6, markers of chronic inflammation.67 In addition, exercise helps to promote weight loss, lowers cholesterol levels and improves insulin sensitivity, functional capacity and quality of life.68-70
The American College of Sports Medicine recommends a multi-component exercise programme (strength, endurance, flexibility, and balance) to maintain fitness.71 Resistance training stimulates protein synthesis, increases muscle mass and strength and improves physical functioning in the elderly.72 The combination of different aerobic exercises and progressive resistance training additionally decreases abdominal and visceral fat while also improving insulin sensitivity, and thus is considered to be the optimal strategy for these patients.
An exercise programme should be individually tailored to each patient according to their comorbidities and degree of disability. To prevent possible musculoskeletal injuries and encourage adherence, the intensity and duration of the sessions should be gradual. Even very elderly or frail people can participate in exercise programmes under close supervision.
Pharmacological treatment
There are not many pharmacological options for the treatment of obesity; many drugs have been withdrawn from the market due to misuse or the occurrence of side effects. For example, following the publication of the results of the SCOUT (The Sibutramine Cardiovascular Outcomes Trial) study, which evaluated the cardiovascular consequences of weight loss with and without sibutramine in 10,744 obese patients aged over 55 who had high cardiovascular risk, it was found that the risks of using sibutramine were greater than its potential benefits, as such the drug was withdrawn from the market.73
Treatment with antiobesity medication is indicated in patients with a BMI greater than or equal to 30 kg/m2 or in patients with a BMI greater than 27 kg/m2 and other associated pathologies who have not been able to lose weight with dietary measures. Its efficacy and safety in people aged over 65 has not been studied in-depth.
The use of liraglutide, a human glucagon-like peptide-1 analogue (GLP-1), has recently been approved in Europe for treating excess weight together with diet and exercise. However, there are no data on its efficacy and safety in the elderly, so it is not recommended for people who are aged over 75.74
Bariatric surgery
Another therapeutic option that is becoming increasingly popular in the elderly population is bariatric surgery. This treatment is one of the most effective long-term measures for the treatment of morbid obesity and has obtained significant reductions in the comorbidities associated with obesity.6 However, it is not suitable for all patients. There is current unanimity that bariatric surgery should be limited to patients who have a BMI greater than 40 kg/m2, or those with a BMI of 35 to 39.9 kg/m2 and major comorbidities, such as heart failure, type 2 diabetes mellitus, high blood pressure or sleep apnea. Candidate patients should undergo a thorough preoperative and postoperative evaluation, carried out by a multidisciplinary team that includes nutritional experts.
Obesity has significant functional implications in the over-65 population because it aggravates the deterioration associated with age. The presence of comorbidities, together with a loss of lean mass and bone tissue that these patients suffer, increases their functional deterioration and raises the risk of disability, hospitalisation and mortality.
There is a synergy between the increase of abdominal fat mass and decrease of muscular tissue, and when both coexist in the same individual their negative consequences are augmented. For this reason, a patient’s weight, their body composition and the accumulation of intra-abdominal fat should be taken into account when assessing their nutritional status.
The priority objective of any weight reduction programme whose focus is the elderly is to preserve muscle mass. An increase in lean mass in these patients will result in an increase in insulin sensitivity, which will contribute to reducing their cardiometabolic risk and an improvement in muscle strength, which will, in turn, result in a lower risk of falls and thus mortality.
As in any age group, the therapeutic strategy par excellence in these patients is a lifestyle change, which includes changes in diet and increased exercise. In obese, elderly patients, treatments must take account of their specific particularities, especially in regard to loss of lean mass, such that any diet prescribed to them should ensure weight loss while minimising any loss of lean mass. To do this, the calorie intake should be reduced by maintaining a high protein intake (1.2 to 1.5 g per kg per day). Which can be achieved with certain very low-calorie diets. Considering its conservative effect on lean mass, these diets, accompanied by a multi-component exercise programme, are a valid option for a weight loss strategy for elderly subjects.
No conflict of interest declared.

©2017 Sajoux, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.