eISSN: 2473-0815


Research Article Volume 5 Issue 2
1Department of Endocrinology and Metabolism, Concord Repatriation General Hospital, Australia
2Sydney Medical School, University of Sydney, Australia
3Metabolic Rehabilitation Program, University Medical Clinic of Camden, Australia
Correspondence: Ramy Bishay, Department of Endocrinology, Metabolism, Level 6, Concord Hospital Medical Centre, Concord, NSW 2139, Australia, Tel +61(02) 9767-5000
Received: June 23, 2017 | Published: July 25, 2017
Citation: Bishay RH, Kormas N. Self-empowerment and health outcomes in obese adults with type 2 diabetes following completion of a multi-disciplinary metabolic rehabilitation program. Endocrinol Metab Int J. 2017;5(2):197-203. DOI: 10.15406/emij.2017.05.00117
Introduction: Type 2 diabetes mellitus (T2DM) is a worldwide epidemic yet; self-empowerment remains an important yet under-utilised aspect of daily self-care in patients with T2DM.
Methods: We retrospectively administered the validated Diabetes Empowerment Scale (DES) to evaluate self-empowerment in 42 obese, adult patients with T2DM who completed a minimum 1-year participation in an intensive multi-disciplinary metabolic rehabilitation program. We sought to determine correlations with clinical outcomes in nine cardiometabolic parameters collected at baseline and subsequently 6-monthly till 30-months.
Results: Over 87% of patients attended ≥3sessions/week, with 95% enrolled up to 30-months. Patients indicated a moderately high level of empowerment both globally and in all three DES domains. Subscale III (setting and achieving diabetes goals) was significantly and inversely correlated with % change in waist circumference (WCC) at 12-months (R=-0.337, P=0.03) and % reduction in diastolic blood pressure at 24-months (r=-0.381, P=0.01). Subscale II (assessing dissatisfaction and readiness to change) was positively correlated with duration of diagnosis (R=0.354, P=0.02). Furthermore, the number of exercise sessions attended was correlated with decreased 12-month % change in weight (r=-0.320, P=0.04) and glycosylated haemoglobin (HbA1c) (r=-0.344, P=0.03). Significant reduction was achieved in as early as 6-months for weight (-4.2±0.9%, P<0.001), BMI (-3.9±0.9%, P=0.002) and WCC (-2.8±0.7%, P=0.004) and continued to decrease at 30 months (-8.6±1.4%, P<0.001; -8.4±1.5%, P<0.001; -5.0±1.3%, P<0.001, respectively). Concurrent with weight reduction, significant improvement in HbA1c was also observed early at 6-months (-8.5±1.6%, P<0.001) with maximum benefit at 24-months (-10.1±2.2%, P<0.001). High-density lipoprotein cholesterol also reached a maximal increase at 24months (10.6±4.3%, P=0.049).
Conclusion: Setting and achieving goals was associated with reductions in waist circumference and blood pressure. The role of empowerment-based intervention in conjunction with intensive multi-disciplinary rehabilitation on health outcomes in these patients remains under-researched and under-utilised in clinical practice.
Keywords: type 2 diabetes mellitus, self-empowerment, diastolic blood pressure, diabetes distress scale.
T2DM, type 2 diabetes mellitus; PAID, problem areas in diabetes; DES, diabetes empowerment scale; MRP, metabolic rehabilitation program; WCC, waist circumference; BMI, body-mass index; sBP, systolic blood pressure; dBP, diastolic blood pressure; HDL, high-density; LDL, low-density lipoprotein; DDS, diabetes distress scale.
Type 2 diabetes mellitus (T2DM) is a global pandemic and despite increased understanding of its pathophysiology and the rapid advent of new pharmacotherapies, achieving target glycaemic control and obviating the development of micro- and macrovascular complications of diabetes is reached in only a minority of patients.1 There is strong evidence suggesting the importance of empowerment, as a major factor in improving glycaemic control in patients with T2DM.2‒6 Self- or patient-empowerment (used interchangeably) in T2DM can be broadly defined as equipping, enabling, assisting, supporting and encouraging people with diabetes to effectively and confidently participate in diabetes self-management.7
A variety of psychometric tests were developed in the past twenty years to evaluate the affect of psychological factors for T2DM, such as Problem Areas in Diabetes (PAID),8 Diabetes Knowledge Test,9 Diabetes Care Profile,10 Diabetes Distress Scale (DDS)11 and Self-Efficacy of patients with Type 2 Diabetes Scale (SE-Type 2).12 A widely studied and validated inventory known as the Diabetes Empowerment Scale (DES), firstcreated in 2000 as a proxy to the popular Diabetes Care Profile,10 was shown to reliably measure self-empowerment and overall assessment of diabetes related psychosocial self-efficacy.13 It is one of the few instruments available currently to measure the concept of empowerment in diabetes care and possesses strong internal consistency (α=0.96). Previous clinical studies have demonstrated a positive effect on DES scores following a 6-week problem-based patient education program.14 In another study, the DES was used to assess psychosocial self-efficacy of Veterans attending a diabetes education program successfully.15 Currently, the DES has been successfully adapted to several populations13,16,17 and has been positively correlated with improvement in HbA1c [7.18] though it is unknown if there are any other correlations with other physiologic parameters.
A multidisciplinary, intensive lifestyle interventional program named the Metabolic Rehabilitation Program (MRP) was created in 2003 in Sydney Australia, as a non-surgical weight loss clinic for obese adults with T2DM. We sought to retrospectively assess the relationship between levels of self-empowerment and clinical outcomes achieved in a small population of obese adults with T2DM patients following completion of an intensive multidisciplinary interventional program. We hypothesized that higher self-empowerment would correlate positively with achieved improvements in metabolic parameters in patients with T2DM who have completed a multi-disciplinary non-surgical metabolic rehabilitation program.
Metabolic rehabilitation program
We previously described the integrated model of the multidisciplinary MRP in detail.19,20 Briefly, the MRP commenced study enrollment of patients from 2007 to 2011 who fulfilled two criteria: (1) BMI ≥35kg/m2; (2) and a diagnosis of T2DM. Patients were excluded if they had cardiorespiratory disease precluding intensive exercise. Patients committed for a minimum of 6-months though the majority of patients continued the program for over 1year. The MRP combined both clinical consultations (endocrinologist specializing in obesity management, dietetics, diabetes educators, psychologists) and physiotherapists, exercise physiologists and personal trainers overlooking supervised exercise sessions. The minimum level of participation was attendance at three exercise sessions per week for duration of 45 minutes each session, and consisted of both weight-bearing and aerobic exercises at a mild-to-moderate level. Patients were prescribed exercise sessions for off-gym days to achieve 315minutes of total exercise per week.
Patient recruitment
This was a retrospective study that included all patients enrolled in the MRP who met the minimum participation criteria. This study adapted the validated DES to gauge the self-efficacy and self-determination in obese T2DM patients. Patients who completed the rehabilitation program were contacted to complete the DES. Demographic and physiologic parameters were collected before enrollment and 6-monthly until completion or discharge from the program for correlative analyses with DES. The physical parameters collected included weight, waist circumference (WCC), body-mass index (BMI), HbA1c, systolic blood pressure (sBP), diastolic blood pressure (dBP), high-density (HDL) and low-density lipoprotein (LDL) cholesterol, and triglycerides (TRI). All protocols and procedures were approved by the local human research ethics committee.
Diabetes Self-Empowerment Scale (DES)
The DES is a 28-item questionnaire based on completed items, with each item selected either as “strongly agree” (5points), “agree” (4points), “neutral” (3points), “disagree” (2points), and “strongly disagree” (1point). The scores are summed for each subscale, with the total divided by the number of items (in this case 10) in the subscale. The resulting value is the score for that subscale. An overall score for the DES is calculated by adding all of the item scores and dividing by 28.7,18
Statistics
All data are expressed as mean ± standard error of the mean (SEM). Percent changes in physiologic parameters are percentage change from baseline. Significant differences in the physical parameters versus baseline means were determined using repeated measures one-way analysis of variance (ANOVA). Pair-wise comparisons between the different time points were calculated using the Holm-Sidak method. Correlations between physical parameters, DES and DAQ scores were determined by Spearman's correlation coefficient on ranks for non-parametric distribution. Normality of distribution of the data was tested using the Shapiro-Wilk method. Linear regression analysis was performed with physical parameters as the dependent variable and either the DES scores as the independent variable. All statistical analyses were performed using SigmaStat and SigmaPlot statistical software (Systat Software Inc., CA and USA). A power of 0.80 was used for all linear regression analyses. P < 0.05 was considered statistically significant.
Patient characteristics
Fourty-two patients (63%) were recruited into the study (Figure 1). Patient characteristics were summarised by our group previously20 (reproduced with permission, Table 1). There was an approximate equal distribution of male (45%) and female (55%) study participants with an average age of 59.1±9.4 years (range 33–72years). The highest level of education achieved by nearly half of patients was up to year 10 (42.8%) with the remainder achieving some form of higher education. The average time from diagnosis of T2DM was 10.5±7.2 years (range 1-34years). The majority of patients were on oral hypoglycaemic agents (metformin and/or sulphonylurea, 80.9%) with only approximately one third receiving either insulin alone or insulin and oral combination therapy. The average number of exercise sessions attended per week was 4.1±0.9, with over 94% of patients attending an average of ≥2sessions/week and 87% of patients attending an average of ≥3 sessions/week. Due to the small numbers, subgroup analyses on those who attended less or more than the minimum 3 exercise sessions was not possible.
Self-Empowerment is associated with cardiometabolic outcomes
The maximum score in an item on the DES is 5 (‘strongly agree’) and the minimum is 1 (‘strongly disagree’). The mean for subscale I (managing the psychosocial aspects of diabetes) was 3.8±0.07, which was significantly higher than the mean of subscale II (assessing dissatisfaction and readiness to change, 3.6±0.08, P=0.005). The mean of subscale III (3.8±0.09, setting and achieving diabetes goals) was similarly higher than subscale II (P=0.005). The global DES score was 3.7±0.07 (Figure 2A). There was a significant positive relationship between DES subscale III (setting and achieving diabetes goals) and % reduction in WCC (R=-0.337, P=0.03) (Figure 2B) and % reduction in dBP at 24-months (r=-0.381, P=0.01). Interestingly, we found that patients who were diagnosed with a longer duration of T2DM correlated with greater weight loss at 12 (r=-0.396, P=0.01) and 18-months (r=-0.451, P=0.005), with a positive liner relationship at 18-months (R=-0.468, P=0.003). Correlation with % change in BMI showed similar findings across the same time points as the % weight change data (Figures 2C&D). Furthermore, subscale II of the DES (dissatisfaction and readiness to change) correlated positively with duration of diagnosis (R=0.354, P=0.02, Figure 2E) but negatively correlated with % change in HDL at 18-months (R=-0.375, P=0.02).

Figure 2 E. Time since diagnosis of T2DM was positively correlated with DES subscale II (assessing dissatisfaction and readiness to change).
Metabolic rehabilitation achieves significant, durable reductions in cardiometabolic parameters
Significant reduction in anthropomorphic measurements (Table 2) was achieved as early as 6-months (weight, -4.2±0.9%, P<0.001; BMI, -3.9±0.9%, P=0.002; WCC, -2.8±0.7%, P=0.004) and continued to decrease at 30 months versus baseline (weight, -8.6±1.4%, P<0.001; BMI, -8.4±1.5%, P<0.001; WCC, -5.0±1.3%, P<0.001). Patients also lost weight when compared with 6-month data. Similar results were obtained for BMI. Waist circumference followed similar trends to weight loss and BMI in the first year of rehabilitation but stabilized thereafter. Percentage reduction in HbA1c was also achieved at 6-months (-8.5±1.6%, P<0.001) and remained stable at 30-months. Maximum benefit was achieved at 24-months (-10.1±2.2%, P<0.001) when compared with baseline. Systolic and dBP showed mixed results, with significant reductions achieved after 1-year (sBP, 6.4±1.99%, P=0.015; dBP,-6.6±2.0%, P=0.008), but did not achieve significance beyond 1-year due to a rebound increase at 12-months. High-density lipoprotein cholesterol increased steadily from baseline, reaching a maximum increase at 24-months (10.6±4.3%, P=0.049). Low-density lipoprotein and TRI cholesterol showed similar improvements at 30-months (LDL,-10.0%±7.3%; TRI,-12.9±6.6%).
Baseline Parameters |
Mean + SEM |
Male |
n = 21 (45%) |
Female |
n = 26 (55%) |
Duration of Diagnosis (Years) |
10.5 + 7.2 (range 1-34) |
Weight (kg) |
104.7 + 19.9 |
Height (m) |
1.67 + 0.1 |
BMI (kg/m2) |
37.6 + 5.7 |
WCC (cm) |
113.4 + 12.6 |
EBW |
35.0 + 16.6 |
%HbA1c |
8.2 + 1.6% |
sBP (mmHg) |
137.0 + 18.6 |
dBP (mmHg) |
79 + 9.1 |
HDL-c, mmol/L |
1.26 + 0.64 |
LDL-c, mmol/L |
2.53 + 0.9 |
TRI, mmol/L |
2.07 + 1.0 |
% Anti-Lipid Medications |
66% (n = 31) |
% Anti-Hypertensive Medications |
74% (n = 35) |
% OHGs |
81% (n = 38) |
% Insulin +/- OHGs |
25% (n = 12) |
% with Metabolic Syndrome |
100% (n = 47) |
Table 1 Baseline Data of Participants in the Metabolic Rehabilitation Progra
BMI: Body Mass Index; WCC: Waist Circumference; EBW: Excess Body Weight; %HbA1c: Glycosylated Hemoglobin; sBP: Systolic Blood Pressure; sBP: Diastolic Blood Pressure; HDL-cL High-Density Lipoprotein Cholesterol; LDL-c: Low-Density Lipoprotein Cholesterol; TRI: Triglycerides; OHGs: Oral Hypoglycaemic Medications
All data are means + SEM.
We evaluated the psychosocial influences on patients’ understanding of diabetes. Our results revealed that patients who felt more comfortable asking their doctor questions about diabetes were more confident in their knowledge of the disease (r=0.367, P=0.02, Figures 3A&3C) and this relationship could be predicted linearly (R=0.417, P=0.007, Figure 3D). Higher scores on the question of patients’ knowledge of diabetes and its treatment were negatively correlated with % change in sBP at 30-months (r=-0.370, P=0.03). Interestingly, most patients felt that having T2DM rarely affected their activities of daily living (Figure 3B). Self-reported scores of one’s ability to fit diabetes into a positive lifestyle are shown in Figure 3E. Furthermore, most patients (81%) felt that the MRP helped them lose weight (Figure 3F).
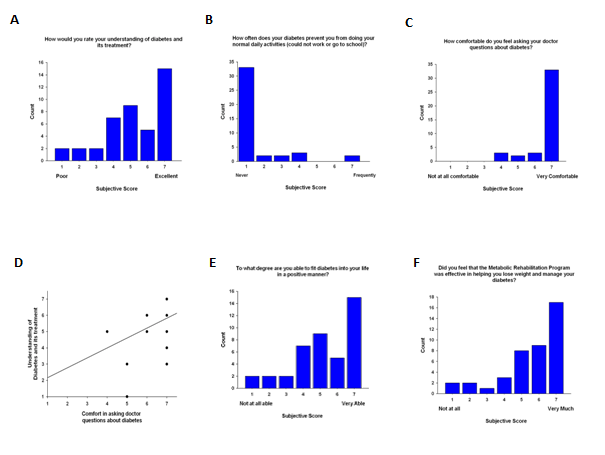
Figure 3 Diabetes attitude items. A. Nearly half of patients (48%) rated their understanding of diabetes and its treatment as 6 or higher with 36% rating either a 4 or 5. Higher scores on the question of patients’ knowledge of diabetes and its treatment were negatively correlated with % change in sBP at 30 months (r = -0.370, P = 0.03). B. Seventy-nine percent (n = 33) of patients rated that their diabetes never affects their ability to perform normal daily activities whereas only 5% (n = 2) scored ‘frequently.’ C. The majority of patients (79%, n=33) were comfortable in asking their doctor questions about diabetes. Only 7% (n = 3) rated a mediocre score of 4. D. Patients who felt comfortable asking their doctor questions about diabetes were more confident in their knowledge of the disease (R = 0.417, P = 0.007). Paucity of data points is due to the significant overlap between the two parameters. E. Self-reported scores of one’s ability to fit diabetes into a positive lifestyle showed a variable distribution of responses among patients, with a majority reporting ‘very able’ (n = 15, 36%) and 31% (n=13) scoring 4 or below. F. Patients’ overall perceived that the rehabilitation program helped them lose weight and manage their diabetes. The majority of patients (81%) gave a score higher than 5, with 62% submitting a score of 6 or higher.
Higher achieved education correlated with greater % weight loss at 6-months (R=-0.360, P=0.02) and greater number of attended exercise sessions correlated with more 12-month % weight loss (r=-0.320, P=0.04) and % change in HbA1c (r=-0.344, P=0.03). Education was correlated only with % change in waist circumference at 6-months (r=-0.360, P=0.02). Six-month data fell short of reaching significance (P=0.057). Respondents noted the affect of diabetes on performance of daily activities was negatively correlated with % change in HDL from 12-months onwards (mean R=0.373, P=0.001).
Our data demonstrate that patients enrolled in an intensive, multi-faceted non-surgical rehabilitation program achieved moderate reduction in weight (-8.5%), BMI (-8.4%), and waist circumference (-5.0%), improved glycaemic control (-10.1% reduction in HbA1c) and higher HDL cholesterol levels (±10.6%) at 30-months; smaller improvements were achieved in blood pressure (sBP, -6.4%; dBP,-6.6%), LDL cholesterol (-10%) and triglycerides (-12.9%) (Table 1). The impressive reduction in HbA1c in this study is substantial given that the UKPDS demonstrated that for each 1% reduction in HbA1c, there was a 21% reduction in deaths related to diabetes, 14% reduction in the incidence of myocardial infarction, and 37% reduction in microvascular complications.21 Furthermore, in the Look AHEAD (Action for Health in Diabetes) trial, the intensive lifestyle intervention arm cohort maintained greater reductions in weight, fitness, HbA1c, sBP, dBP and lipid profile versus the diabetes support and education group at 4-years.22
Parameter |
Baseline |
6 m (n=42) |
12 m (n=42) |
18 m (n=39) |
24 m (n=40) |
30 m (n=35) |
Weight % change |
103.6 + 3.1 |
-4.3 + 0.9* |
-7.1 + 1.0** |
-6.4 + 0.9** |
-7.8 + 1.2** |
-8.5 + 1.4** |
BMI % change |
37.2 + 0.8 |
-3.9 + 1.0* |
-7.1 + 1.0** |
-6.4 + 0.9** |
-7.7 + 1.2** |
-8.4 + 1.5** |
WCC % change |
112.0 ± 1.9 |
-2.8 + 0.7* |
-5.1 + 0.7** |
-3.9 + 0.8* |
-5.1 + 1.0* |
-5.0 + 1.3* |
%HbA1c % change |
8.1 ± 0.2 |
-8.5 ± 1.6* |
-10.0 ± 2.2* |
-9.0 ± 2.2* |
-10.1 ± 2.2* |
-8.3 ± 2.6* |
sBP % change |
136.9 ± 2.9 |
-2.0 ± 1.9 |
-6.4 ± 2.0* |
-1.7 ±2.2 |
-4.2 ± 2.3 |
-5.4 ± 2.4 |
dBP % change |
79.7 ± 1.4 |
-4.4 ± 2.0 |
-6.6 ± 2.0* |
-1.3 ± 2.2 |
-4.3 ± 1.9 |
-6.2 ± 2.4 |
LDL % change |
2.5 ± 0.2 |
-7.0 ± 3.0 |
-2.2 ± 4.2 |
-9.9 ± 6.1 |
-3.7 ± 7.6 |
-10.0 ± 7.3 |
HDL % change |
1.3 ± 0.1 |
2.5 ± 3.0 |
8.2 ± 3.7 |
8.3 ± 4.3 |
10.6 ± 4.3* |
7.7 ± 3.9 |
TRI % change |
2.1 ± 0.2 |
-8.0 ± 4.5 |
-4.7 ± 4.7 |
-14.1 ± 5.9 |
-13.9 ± 5.9 |
-12.9 ± 6.6 |
Table 2 Metabolic Rehabilitation Achieves long-term weight loss and improvements in cardiometabolic parameters
All data are reported as mean + SEM. n = the number of patients. *P<0.05 versus baseline; **P<0.05 versus 6-month values.
In Australia, only two centers employing multi-faceted, publically-funded long-term interventional centers for obese adult T2DM patients with a supervised exercise component have been established, both by our group. The majority of patients seem to enjoy the program and feel that it helped them lost weight and manage their disease (Figure 3F). Data from twenty longitudinal cohort studies and six large-scale diabetes prevention intervention trials in adults with impaired glucose tolerance or at high risk of cardiovascular disease unanimously reflected the beneficial effect of moderate physical activity (approximately 150 minutes per week) in reducing the risk of developing the T2DM by nearly 20-30%.23‒26 Substantial amelioration of risk (40-60%) is achieved in pre-diabetic patients however with rigorous lifestyle intervention counseling for physical activity, nutrition, and weight loss.24
Differences in patient self-empowerment subscales
The ability and motivation to self-manage diabetes was measured using the DES–one of a few currently available instruments able to measure the concept of empowerment in diabetes care.13 We discovered that patients in this study reported a moderately high level of global self-empowerment (Figure 2A), with significantly lower reports in subscale 2 (assessing dissatisfaction and readiness to change) compared with the other global and subscale scores. The scores obtained in this cohort are comparable to other larger studies, such as those described in the initial cohort reported by Anderson et al. where mean scores for subscale (I) were 3.91±0.70[1.44–5.00]; (II) 3.96±0.53[1.78–5.00] and (III) 3.96±0.62[1.80–5.00])18 as well as a cohort of Icelandic patients with diabetes (mean DES score 3.68).27 In general, higher scores were associated with more positive clinical outcomes and health behaviours though no strict cut-off or value has been proposed. Specifically, the DES subscale II assesses patients’ perceived ability to identify aspects of caring for diabetes that they are dissatisfied with and their ability to determine when they are ready to change their diabetes self-management plan.18 This possibly reflects that patients are either unaware of negative diabetes-related stresses in their life or are reluctant to change them. This specific subscale is of particular interest for future prospective and randomized trials as it may reflect a patient’s ability for behavioural change.
Waist circumference, weight loss and self-empowerment
We found a positive correlation with patients who lost central, abdominal fat and DES subscale III (assessing setting and achieving diabetes goals) (Figure 2B). As waist circumference is strongly associated with increased cardiovascular events, mortality, visceral fat, and insulin resistance,28 and that abdominal fat is physiologically resistant to fatty oxidation, it may reflect the degree of lifestyle change in patients, which is primary substrate for assessment in DES subscale III (i.e.,a patients’ perceived ability to set realistic diabetes goals and reach them by overcoming the barriers to achieving their goals).18 Though we found that the decreases in waist measurements stabilized after 1-year, we presumed that this was likely due to increased abdominal muscle mass. We also did not directly identify any significant correlations with DES scores and weight loss. Ideally, patients would be asked to complete the DES during or shortly following discharge from the program, when self-esteem, empowerment and psychosocial health status are likely to be the highest.5 Unfortunately, no baseline DES scores were obtained in this cohort. Naturally the DES scores were more reflective of patients’ current psychosocial state rather than at the time of rehabilitation. The affect of this limitation would likely limit the positive findings observed in our study as exercise and weight reduction are often associated with high motivational levels and generally greater psychosocial health status. Nonetheless, the DES global and subscale scores just failed to reach statistical correlative significance and all regression analyses showed the expected direction, indicating that higher empowerment levels may still be related to achieved weight loss.
We discovered a significant association between improved weight loss and duration of diagnosis, perhaps reflecting the frustration and desperation for change in older patients with diabetes who have often tried multiple diet and exercise regimens in the past. A study from 2004 comprising Arabic-speaking T2DM patients revealed that duration of diabetes remained an independent predictor of ‘lower satisfaction’ and higher ‘impact on quality of life’ after controlling for patient’s demography and diabetes related factors as assessed by the Diabetic Quality of Life survey.29 This would seem consistent with our results showing a positive correlation between DES subscale II (assessing dissatisfaction and readiness to change) with duration of T2DM (Figure 2E). The same aforementioned study by Girgis et al.29 also showed that higher levels of HbA1c were correlated with lower satisfaction scores and demonstrated glycaemic control as an independent predictor for patients’ higher satisfaction with life,29 though our results failed to achieve significance with HbA1c analyses and DES scores. Previously, DES scores were shown to be positively correlated with improvement in HbA1c.7,18 The lack of significance may reflect the insensitivity of the DES to correlate with changes in glycaemic control in our population or more likely the limited power in our analyses as a result of the small patient cohort studied. Nonetheless, the DES global and subscale scores just failed to reach statistical significance and the preliminary data suggests that higher empowerment levels may still be related to improved glycaemic control. Interestingly, the Copenhagen Type 2 Diabetes Rehabilitation Project30 -a randomized control trial of a small population of patients with T2DM undergoing a rehabilitation program consisting of empowerment-based education, supervised exercise and dietary intervention – found similar reductions at 1-year in weight (2.2 vs 1.6kg), waist circumference (2.0 vs 1.6cm), HbA1c (0.2 vs 0.4%), sBP (6 vs 3mmHg) and dBP (4 vs 3mmg) between the rehabilitation group versus the individual outpatient primary care group, suggesting both models of care are of similar efficacy.31 Larger seminal trials, however, such as the Look AHEAD trial,32 clearly showed early superiority of the intensive lifestyle intervention arm versus the conventional treatment arm, with the former group having lost >5% in more than 50% of patients despite a lack of clear cardiovascular benefit at 9.6-yrs of follow-up.22‒32
Our results showed that patient comfort in relation to asking their doctor questions about diabetes is correlated with greater understanding of T2DM and its treatment (Figures 3A, C, D). Previous studies showed the DES to correlate significantly in the expected direction with patients’ self-reported comfort in asking questions of their physician and their self-reported positive adjustment to diabetes.7 Furthermore, our analysis supports many previous studies noting the strong association between education and prevalence and severity of T2DM and other chronic diseases.33,34 Nearly half of our patients achieved up to year 10 (42.8%) and this may reflect a number of other related factors such as lower socioeconomic status, lack of access to healthier fresh food, and less affordability of medications, gym facilities, and specialist visits. A study by Saydah et al.33 revealed that patients who possessed less than a high school education had a twofold risk higher mortality from diabetes, after controlling for other confounders, compared with adults with a college degree or higher education level.33
Study limitations
Main study limitations included the retrospective nature of the analysis as well as the reliance on self-reported levels of empowerment following completion, rather than at the time, of rehabilitation. The study design would likely underestimate the relationship between self-empowerment and improvement in cardiometabolic parameters (e.g., weight loss and DES scores). The type and amount of pharmacological treatment for comorbid conditions (e.g., hyperlipidaemia, hypertension) and pharmacological unloading were also not evaluated; the latter may have confounded the rebound in blood pressure measurements seen after 12-months. Finally, selection bias may have played a role in our study as patients who enrolled in our program can be said to be already highly motivated individuals; this presents an interesting avenue for further research to determine the innate empowerment levels of rehabilitation versus standard clinic patients.
Obese adults with T2DM can achieve moderate amelioration of metabolic and cardiovascular risk factors within a multi-faceted, intensive, non-surgical interventional program aimed at reduction of weight, improvement of fitness, diet modification and psychosocial support at 30-months. Retrospective analyses revealed empowerment – specifically the subscale assessing ‘setting and achieving diabetes goals’ – to be positively correlated with reduction in waist circumference and blood pressure. Subscale II of the DES ‘assessing dissatisfaction and readiness to change’ correlated positively with duration of diagnosis and patients who have had T2DM for a longer period of time were more likely to lose more weight during metabolic rehabilitation. Future randomized studies are needed to determine if non-surgical metabolic rehabilitation is superior to current best practice diabetes clinics based on long-term health outcomes already described and whether self-empowerment, assessed at baseline, is predictive of favourable clinical outcomes.
RB researched, drafted and edited the manuscript and approved the final version for submission.
RB researched, drafted and edited the manuscript and approved the final version for submission; NK provided the data for the cases and edited the manuscript and approved the final version for submission.
The authors declare that there is no conflict of interest.

©2017 Bishay, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.