eISSN: 2575-906X


Macroinvertebrates owing to their wide variation of response to environmental changes have been extensively utilized to evaluate the water quality and health of the aquatic ecosystems. Seasonal sampling of the macroinvertebrates can indicate the effects of anthropogenic activities on the community. The aim of the present contribution is to monitor species composition and abundance and utility of macroinvertebrates in assessing the health of high altitude (3,075 masl.) wetland, Dodi Tal of Garhwal Himalaya, India. A total of 29 taxa of macroinvertebrates belonging to Trichoptera, Ephemeroptera, Plecoptera, Diptera, Coleoptera, Oligochaeta and Gastropoda groups were recorded from the high altitude wetland, Dodi Tal. Most potential species such as Heptagenia sp. Ephemerella sp. (Ephemeroptera), Perla sp.; Isoperla sp. (Plecoptera) and Orthrotrichis sp. Mystacides sp. (Trichoptera) , Enchytreaus sp. (Oligochaeta) were identified as excellent bioindicator on the basis of their abundance for assessing the health of the high altitude wetland Dodi Tal. Shannon Wiener diversity index and Canonical Correspondence Analysis (CCA) of macroinvertebrates were analyzed to determine the effect of physico- chemical variables on the abundance of these taxa of macroinvertebrates.
Keywords: high altitude wetland, macroinvertebrates, bio-indicator, canonical correspondence analysis, garhwal himalaya
AT, air temperature; WT, water temperature; TUR, turbidity; TDS, total dissolved solid; CO, conductivity; ALK, alkalinity; FCO2, free CO2; DO, dissolved oxygen; BOD, biological oxygen demand; CH, chloride; TOH, total hardness; CA, calcium; MA, magnesium; NI, nitrates; PHO, phosphates; SUL, sulphates; NA, sodium; K, potassium
Biological monitoring is the systematic use of living organisms or their response to determine the quality of aquatic environment.1 Peckarsky (1997) has stated that different aquatic invertebrates have different tolerances ,such as their zone of habitat, degradation , recovery and clean water, their character and signs of species being found may be their nature of tolerance. Benthic macroinvertebrates in lentic habitats are considered indicators for assessing the health of the ecosystem.3 Benthic macroinvertebrates (bottom feeders) dwelling near the bottom of the water bodies may be particularly potential indicators of watershed health, as they have restricted mobility and are indicators of ecological condition.4,5 Hellawell et al.,6 identified the reasons for preferring benthic macroinvertebrates to other groups. Macroinvertebrates are major groups of aquatic invertebrates with long lives, so macroinvertebrates can be used for a long time to assess water quality The group is heterogeneous and a single sampling technique may catch a considerable number of species. Therefore some species will respond to a particular environmental change.
High altitude wetlands of the Himalaya are very important for providing drinking water for trekkers, hikers, shepherd and their cattle, wild animals and tourists. Thus, the health of these freshwater ecosystem is of paramount importance. Armitage et al.,7 and Rosenberg et al.,8 have reported that macrozoobenthic species are differentially sensitive to any biotic and abiotic factor in the environment and can be used an indicator of an aquatic ecosystem. Lang et al.,9 reported that the macroinvertebrates can act as potential ecological indicator. Barbosa et al.,10 contributed on the diversity of benthic macro-invertebrates as an indicator of water quality and ecosystem health of rivers and creeks of Serra do Cipo Brazil. Hodkinson et al.,11 emphasized that the use of terrestrial and aquatic invertebrates as a management tool for monitoring changes in ecosystem with particular references to mountain ecosystem. Sharma et al.,12 contributed on the monitoring of aquatic macroinvertebrates as bioindicator for assessing the health of the wetlands for the Central Himalaya, India. Barman and Gupta reported the aquatic insects as bioindicator of water quality for Assam, North East, India. However, no attempt has been made so far on the monitoring of aquatic macroinvertebrates as bioindicator for assessing the health of the high altitude wetland of Garhwal Himalaya. The present study was aimed at monitoring the macroinvertebrates, as bioindicator for assessing the health of high altitude wetland, Dodi Tal of Garhwal Himalaya.
The study area
A high altitude wetland, Dodi Tal located at an altitude of 3,075 above m.s.l lies between 30o52’31.99” N latitude and 78o31’12.47” E longitude (Figure 1). The Dodi Tal is the important destination of large number of tourists, trekkers, hikers and shepherds. It is a large, somewhat triangular shaped wetland with an approximate length of 248.22 m, width of 151.99 m and depth of 19.97m with a catchment area of about 3.0623 ha. Dodi Tal receives water from precipitation, springs and melting of snow. The Dodi Tal area experiences five distinct seasons viz. the cold winter season (November to January), spring season (February to March), summer season (April to June), southwest monsoon season (July to August) and autumn (September to October). The area remains very cold during winters with severe snowfall; while during summers, it gets a little warm. The air temperature ranges from a minimum of -2oC to a maximum of 20oC.
Regular monthly sampling was undertaken from four sampling sites having variation in microhabitat and level of disturbance between 8:00 to 10:00 hrs period of November, 2014 to October, 2016 except February and March. The Dodi Tal was not accessible during February and March to due heavy snowfall. Five replicates of samples from each sampling sites were taken for the analysis of physico-chemical and biological parameters. The monthly data were pooled for making them seasonal. The physico-chemical parameters like pH, air temperature, water temperature and dissolved oxygen were measured at the wetland. Water temperature was recorded with the help of the Digital Thermometer (-500C to +3000C); Electrical conductivity and pH of the sample were measured with the help of the Toshcon Multiparameter Analyser (Model No. TPC-17). Nitrates, phosphates and sulphates were determined by using the Spectrophotometer (Model –UV-VIS Systronics 117 series). Dissolved Oxygen, total hardness, BOD, alkalinity, Calcium and Magnesium were measured following the methods outlined in Wetzel and Likens (1991) and APHA (1998). Macroinvertebrates colonizing the bottom substrates were collected with the help of the Surber sampler (0.50 mm mesh net). The macroinvertebrates were preserved in 4% formalin. The qualitative analyses of the macroinvertebrates were made with the help of Needham et al.,13 Macan14 Elliot15 Ward et al.,16,17 Edington et al.,18 Mitra,19 Biswas et al.,20 Edmondson,21 Welch,22 Munshi et al.,23 Species diversity index ( ) was calculated using the Shannon Wiener information function.24 Shannon Wiener diversity index was calculated using the following formula:
Diversity index ( ) =
= Shannon Wiener index of diversity; ni = Total no of individual of a species; N = Total no of individuals of all species.
Canonical Correspondence Analysis (CCA) was used to determine the relationship between macroinvertebrates species distribution and physico-chemical variables of wetland. The Canonical Correspondence Analysis was performed using P Aleonotological Statistics (PAST) Software version 2.10.
The mean seasonal physico-chemical characterstics of the high altitude wetland Dodi Tal are presented in Table 1. Seasonally, air temperature, water temperature, turbidity, TDS, conductivity, alkalinity, free Co2, BOD, total hardness, nitrates, phosphates, sulphates, Calcium, Magnesium and Potassium were found maximum in monsoon season and minimum in winter season in Dodi Tal. However, the dissolved oxygen and pH were found maximum in winter season and minimum in monsoon season in Dodi Tal. Concentration of chlorides and sodium were found maximum in summer season and minimum in winter season (Table 1). A total of 29 taxa of macroinvertebrates were recorded from the high altitude wetland, Dodi Tal during the period of study. The percentage composition of macroinvertebrates was represented by Ephemeroptera (14%), Plecoptera (7%), Trichoptera (46%), Diptera (18%), Coleoptera (7%), Oligochaeta (4%) and Gastropoda (4%) during the two year of observations (Figure 2).
|
Environmental variables |
Sites |
Winter |
Summer |
Monsoon |
Autumn |
|
Air temp (OC) |
S1 |
8.00±2.174 |
10.07±2.980 |
11.95±0.650 |
11.45±0.350 |
|
S2 |
7.87±2.347 |
9.80±2.934 |
11.75±0.650 |
11.40±0.500 |
|
|
S3 |
7.90±2.273 |
9.93±2.963 |
11.85±0.650 |
11.50±0.400 |
|
|
S4 |
7.90±2.325 |
10.07±2.980 |
11.95±0.650 |
11.45±0.350 |
|
|
Water temp. (OC) |
S1 |
6.40±2.670 |
8.77±3.126 |
10.25±1.450 |
9.90±1.000 |
|
S2 |
6.33±2.562 |
8.77±3.284 |
10.35±1.550 |
9.65±1.250 |
|
|
S3 |
6.50±2.592 |
8.70±3.159 |
10.25±1.550 |
9.80±1.000 |
|
|
S4 |
6.57±2.546 |
8.77±3.126 |
10.25±1.450 |
9.90±1.000 |
|
|
Turbidity (NTU) |
S1 |
0.12±0.056 |
1.14±0.264 |
1.60±0.190 |
1.12±0.105 |
|
S2 |
0.13±0.052 |
0.96±0.213 |
1.50±0.185 |
1.07±0.075 |
|
|
S3 |
0.18±0.063 |
1.08±0.272 |
1.55±0.190 |
1.10±0.090 |
|
|
S4 |
0.21±0.070 |
1.14±0.264 |
1.60±0.190 |
1.12±0.105 |
|
|
TDS (mg l-1) |
S1 |
35.80±14.961 |
128.43±68.076 |
189.50±51.500 |
65.40±39.600 |
|
S2 |
36.83±14.575 |
117.13±62.300 |
169.00±46.000 |
61.50±36.600 |
|
|
S3 |
37.53±14.539 |
125.37±67.443 |
186.00±50.000 |
62.45±37.250 |
|
|
S4 |
38.63±15.071 |
128.43±68.076 |
189.50±51.500 |
65.40±39.600 |
|
|
Conductivity (µScm-1) |
S1 |
63.43±15.333 |
166.10±87.877 |
386.00±63.000 |
94.15±32.850 |
|
S2 |
64.33±15.633 |
158.90±84.332 |
368.50±67.500 |
85.05±25.950 |
|
|
S3 |
65.23±15.608 |
161.70±85.847 |
378.00±66.000 |
87.60±28.405 |
|
|
S4 |
66.20±15.650 |
166.10±87.877 |
386.00±63.000 |
94.15±32.850 |
|
|
Alkalinity (mg.l-1) |
S1 |
31.50±7.427 |
36.50±7.874 |
46.00±2.500 |
34.00±3.500 |
|
S2 |
32.00±7.427 |
35.40±7.582 |
44.25±2.250 |
32.75±3.750 |
|
|
S3 |
32.50±7.427 |
35.83±7.641 |
44.75±2.250 |
33.50±4.000 |
|
|
S4 |
33.00±7.427 |
36.50±7.874 |
46.00±2.500 |
34.00±3.500 |
|
|
Free CO2 (mg l-1) |
S1 |
2.73±0.437 |
3.59±0.452 |
4.29±0.550 |
2.75±0.110 |
|
S2 |
2.80±0.535 |
3.39±0.223 |
3.74±0.660 |
2.64±0.000 |
|
|
S3 |
2.87±0.634 |
3.52±0.359 |
3.96±0.660 |
2.75±0.110 |
|
|
S4 |
2.95±0.735 |
3.59±0.452 |
4.29±0.550 |
2.75±0.110 |
|
|
Dissolved oxygen (mg.l-1) |
S1 |
11.93±0.499 |
9.93±0.125 |
9.15±0.050 |
9.50±0.600 |
|
S2 |
11.73±0.499 |
10.23±0.125 |
9.60±0.000 |
10.10±0.500 |
|
|
S3 |
11.57±0.464 |
10.17±0.047 |
9.40±0.000 |
9.80±0.600 |
|
|
S4 |
11.33±0.419 |
9.93±0.125 |
9.15±0.050 |
9.50±0.600 |
|
|
B.O. D |
S1 |
0.33±0.094 |
0.53±0.094 |
1.00±0.100 |
0.85±0.050 |
|
S2 |
0.33±0.094 |
0.40±0.082 |
0.85±0.050 |
0.75±0.050 |
|
|
S3 |
0.40±0.082 |
0.50±0.082 |
0.95±0.050 |
0.85±0.050 |
|
|
S4 |
0.40±0.082 |
0.53±0.094 |
1.00±0.100 |
0.85±0.050 |
|
|
pH |
S1 |
7.66±0.169 |
7.15±0.246 |
7.57±0.145 |
6.89±0.100 |
|
S2 |
7.64±0.163 |
7.27±0.232 |
7.68±0.110 |
7.01±0.090 |
|
|
S3 |
7.60±0.166 |
7.22±0.234 |
7.60±0.115 |
6.93±0.065 |
|
|
S4 |
7.54±0.154 |
7.15±0.246 |
7.57±0.145 |
6.89±0.100 |
|
|
Chlorides (mg l-1) |
S1 |
4.73±0.669 |
12.78±1.159 |
7.81±3.550 |
6.39±2.130 |
|
S2 |
8.05±1.771 |
12.78±1.159 |
7.81±3.550 |
6.39±2.130 |
|
|
S3 |
8.05±1.771 |
12.78±1.159 |
7.81±3.550 |
6.39±2.130 |
|
|
S4 |
8.05±1.771 |
12.78±1.159 |
7.81±3.550 |
6.39±2.130 |
|
|
Total Hardness(mg l-1) |
S1 |
20.93±6.979 |
27.00±4.490 |
36.30±2.500 |
24.50±1.300 |
|
S2 |
21.20±7.073 |
25.53±5.076 |
35.50±2.500 |
22.30±2.500 |
|
|
S3 |
21.80±7.073 |
25.93±5.076 |
35.90±2.500 |
23.60±1.600 |
|
|
S4 |
22.13±6.979 |
27.00±4.490 |
36.30±2.500 |
24.50±1.300 |
|
|
Nitrates (mg l-1) |
S1 |
0.02±0.005 |
0.11±0.027 |
0.21±0.015 |
0.10±0.026 |
|
S2 |
0.03±0.005 |
0.08±0.008 |
0.10±0.006 |
0.08±0.011 |
|
|
S3 |
0.03±0.004 |
0.09±0.007 |
0.14±0.020 |
0.09±0.013 |
|
|
S4 |
0.04±0.007 |
0.11±0.027 |
0.21±0.015 |
0.10±0.026 |
|
|
Phosphates (mg l-1) |
S1 |
0.01±0.004 |
0.07±0.074 |
0.09±0.001 |
0.07±0.009 |
|
S2 |
0.02±0.004 |
0.07±0.010 |
0.06±0.001 |
0.07±0.010 |
|
|
S3 |
0.03±0.000 |
0.07±0.000 |
0.09±0.003 |
0.07±0.001 |
|
|
S4 |
0.03±0.004 |
0.08±0.00 |
0.09±0.004 |
0.08±0.012 |
|
|
Sulphates (mg l-1) |
S1 |
0.01±0.004 |
0.05±0.016 |
0.08±0.001 |
0.06±0.007 |
|
S2 |
0.01±0.004 |
0.06±0.060 |
0.06±0.002 |
0.07±0.011 |
|
|
S3 |
0.02±0.000 |
0.06±0.006 |
0.09±0.003 |
0.07±0.001 |
|
|
S4 |
0.02±0.003 |
0.07±0.01 |
0.09±0.002 |
0.07±0.015 |
|
|
Sodium ( mg l-1) |
S1 |
0.75±0.230 |
1.02±0.096 |
0.95±0.110 |
1.00±0.190 |
|
S2 |
0.79±0.221 |
0.82±0.113 |
0.77±0.075 |
0.81±0.180 |
|
|
S3 |
0.91±0.135 |
0.92±0.094 |
0.88±0.090 |
0.91±0.170 |
|
|
S4 |
1.05±0.111 |
1.02±0.096 |
0.95±0.110 |
1.00±0.190 |
|
|
Potassium ( mg l-1) |
S1 |
0.83±0.197 |
1.32±0.054 |
1.40±0.015 |
1.24±0.255 |
|
S2 |
0.95±0.121 |
1.00±0.084 |
0.94±0.030 |
0.98±0.165 |
|
|
S3 |
1.01±0.112 |
1.06±0.073 |
1.07±0.005 |
1.07±0.145 |
|
|
S4 |
1.16±0.056 |
1.32±0.054 |
1.40±0.015 |
1.24±0.255 |
|
|
Calcium (mg l-1) |
S1 |
4.62±0.590 |
5.34±0.553 |
7.09±0.120 |
5.01±0.525 |
|
S2 |
4.70±0.590 |
5.13±0.523 |
6.85±0.040 |
4.85±0.525 |
|
|
S3 |
4.78±0.590 |
5.23±0.537 |
6.97±0.080 |
4.93±0.525 |
|
|
S4 |
4.86±0.590 |
5.34±0.553 |
7.09±0.120 |
5.01±0.525 |
|
|
Magnesium ion (mg l-1) |
S1 |
2.29±1.343 |
3.33±1.101 |
4.54±0.535 |
2.93±0.005 |
|
S2 |
2.31±1.366 |
3.10±1.313 |
4.49±0.635 |
2.49±0.290 |
|
|
S3 |
2.41±1.368 |
3.13±1.295 |
4.51±0.560 |
2.76±0.075 |
|
|
|
S4 |
2.44±1.345 |
3.33±1.101 |
4.54±0.535 |
2.93±0.005 |
Table 1 Seasonal variations ( ± SD) in physico-chemical environmental variables of high altitude wetland, Dodi Tal, Garhwal Himalaya recorded for the period of November 2014 - October 2015
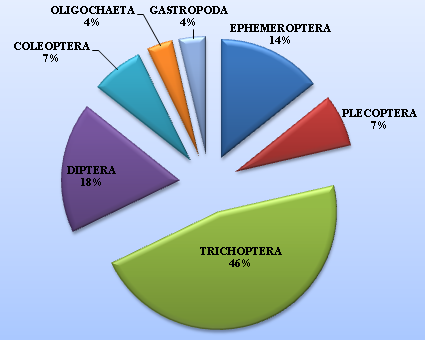
Figure 2 Percentage composition of macroinvertebrates community dwelling the Dodi Tal, Garhwal Himalaya.
Seasonally, the mean density of macroinvertebrates was recorded to be maximum (309.33±8.81ind.m-2) in winter season at S4 and minimum (38.50±2.50 ind.m-2) in monsoon season. Takeda25 reported that Ephemeroptera, Plecoptera and trichoptera (EPT) are the indicator of the good health of the wetland. Ephemeroptera (May flies) are generally considered as indicator species for assessing the quality of wetland water.12 Maximum density of Ephemeroptera was observed (64.00±5.10 ind.m-2) in Dodi Tal. Trichoptera (Caddis flies) was found to be the most dominant (178.33±3.77 ind.m-2) group in winter season (Figure 3).
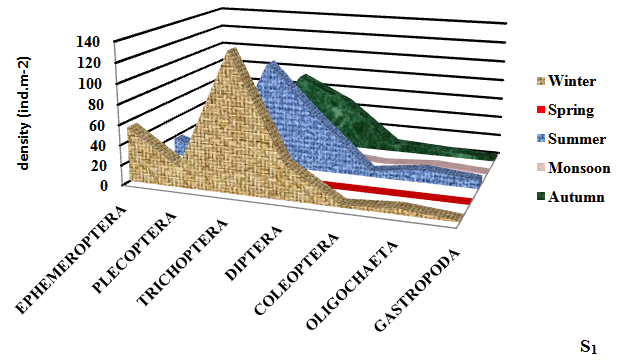
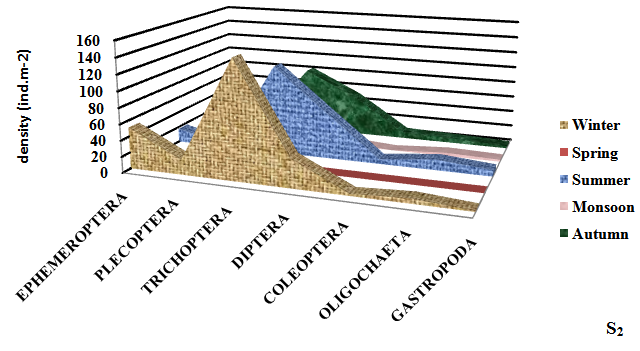
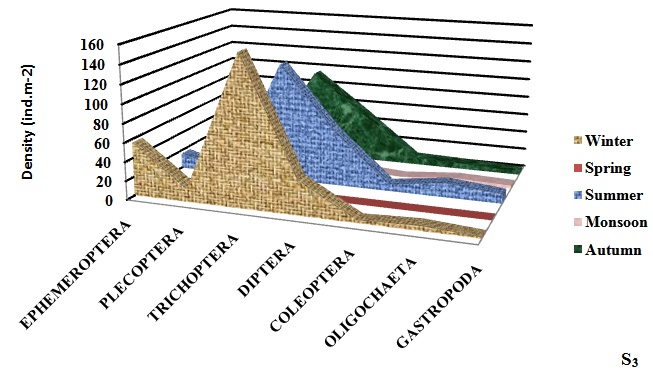
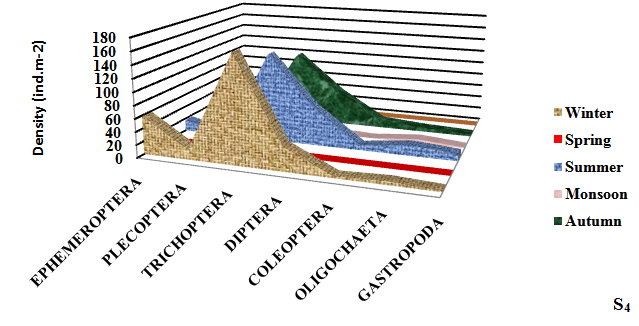
Figure 3 Seasonal variation in density of different groups of macroinvertebrates at different sampling sites of high altitude wetland, Dodi Tal during November 2014-October 2015.
Plecoptera (Stoneflies) mostly prefer cool, clean aquatic habitat. Most stoneflies occur in stony habitats, often within the substrate of leaf packs. Plecoptera are highly sensitive to environmental degradation.26 For this reason, they are often first species to disappear from polluted aquatic habitat. This may be the reason of low density of Plecoptera at the degraded sites (S3 and S4) of Dodi Tal.
Trichoptera (Caddis fly) is one of the most diverse groups found in a broad spectrum of aquatic habitats, their wide diversity occurs in Dodi Tal. The members of Trichoptera belong to functional feeding groups like shredders, grazers and filters feeders.27 Trichoptera was found to be the most dominant (178.33±3.77 ind.m-2) in Dodi Tal. Thus contributed 50 -52% of macroinvertebrates.
Most potential indicators were identified as Heptagenia sp. Ephemerella sp. (Ephemeroptera) Perla sp. and Isoperla sp. (Plecoptera); Hydroptilla sp.; Orthrotrichia sp. and Mystacides sp. (Trichoptera). Perla sp; Isoperla sp.; Orthrotrichia sp. and Mystacides sp. were absent in monsoon season. Abundance of Heptagenia sp. (19.00±2.94 ind.m-2) and Ephemerella sp. (16.33±4.64 ind.m-2) (Ephemeroptera) were found maximum in winter season and minimum (3.00±1.00; 1.50±1.50) in monsoon season due to environmental degradation. Abundance of Perla sp. (7.33±2.62 ind.m-2) and Isoperla sp. (6.67±2.62 ind.m-2) (Plecoptera) reduced; Hydroptilla sp. (24.33±2.49 ind.m-2); Orthrotrichia sp. (20.00±3.27 ind.m-2) and Mystacides sp. (20.33± 0.94 ind.m-2) (Trichoptera) were also found maximum during winter season, which disappeared during monsoon season due to poor health of the ecosystem . Thus, these species can be considered as potential bioindicator.
Diptera (True flies) are tolerant taxa. Maximum diversity of Diptera was found (62.33±11.84 ind.m-2) in summer season at the disturbed site (S4). Abundance of Diptera was found in the of S1<S2<S3<S4 in the present study. High abundance of Diptera at S3 and S4 indicated poor water quality at these sites. Coleoptera (Beetles) play a major role in breakdown of organic material. Maximum percentage of Coleoptera was observed (13.50±1.50 ind.m-2) at S4. Oligocheata and Gastropoda also contributed to total macroinvertebrates of Dodi Tal. Indumathi et al.,28 also observed Oligochaeta as major component of benthic community. Low density of Oligocheata was recorded during winter season and highest in summer season. Similar seasonal trend in abundance of Oligochaeta having high temperature in summer season was reported by Vashisht et al.29 Maximum abundance of Oligochaeta (Enchytreaus sp.) was also found in summer season (16.00±2.94 ind.m-2) in Dodi Tal and low density (7.00±2.94 ind.m-2) in winter season. Thus, this species Enchytreaus sp. (Oligochaeta) can be a potential indicator for assessing the health of Dodi Tal.
Pearson’s correlations calculated between major physico-chemical parameters and macroinvertebrates have been presented. Density of macroinvertebrates density has positive correlation with dissolved oxygen ( r = 0.743, p< 0.05) and negative correlation with the air temperature ( r = -0.665, p< 0.05), turbidity ( r = -0.820, p< 0.01), conductivity( r = -0.890 p< 0.01), total hardness ( r = 0.722, p< 0.05), BOD ( r = -0.866, p< 0.05) , nitrates ( r = -0.832, p< 0.01) and phosphates ( r = -0.846, p< 0.01). Thus, the macroinvertebrates are depleting during high water temperature and turbid water of monsoon season. Ephemeroptera has highly positive correlation with dissolved oxygen (r = 0.960, p< 0.01) and negative correlation with turbidity (r = -0.922, p< 0.01), nitrates (r = -0.886, p< 0.01), phosphates (r = -0.895, p< 0.01), sulphates (r = -0.861, p< 0.01). Plecoptera has highly positive correlation with dissolved oxygen (r = 0.954, p< 0.01) and negative correlation with turbidity (r = -0.869, p< 0.01), nitrates (r = -0.803, p< 0.01), phosphates (r = -0.822, p< 0.01) and sulphates (r = -0.763, p< 0.01). Trichoptera has positive correlation with dissolved oxygen (r = 0.765, p< 0.01) and negative correlation with turbidity (r = -0.854, p< 0.01), nitrates (r = -0.862, p< 0.01) and phosphates (r = -0.876, p< 0.01). Ward et al.,17 suggested that water temperature, depth and substrate composition are the major factors determining the composition and abundance of macroinvertebrates.
Linear regression was calculated between density of macroinvertebrates and physico chemical parameters. Regression plot calculated between physico-chemical parameters and density of macroinvertebrates revealed a positive correlation with the dissolved oxygen (y = 75.361x – 596.32, R2 = 0.551, where y =macroinvertebrates density). However, the macroinvertebrates have negative correlation with air temperature (y = - 24.56x + 433.86, R2 = 0.469), turbidity (y = - 148.51x + 319.27, R2 = 0.671), nitrates (y = - 1751x + 341.87, R2 = 0.0.692), phosphates (y = - 2068x + 343.71, R2 = 0.715) and sulphates (y = - 2039x + 315.17, R2 = 0.534). Macroinvertebrates have negative correlation with turbidity (F value = 16.38, t stat = -4.047), conductivity (F value = 30.40, t stat = -5.514) , nitrates (F value = 18.01, t stat = -4.245) , phosphates (F value = 20.10, t stat = -4.48) and sulphates (F value = 9.19, t stat = -3.032), However, it has a positive correlation with dissolved oxygen (F value = 9.847, t stat = 3.138 (Figure 4).
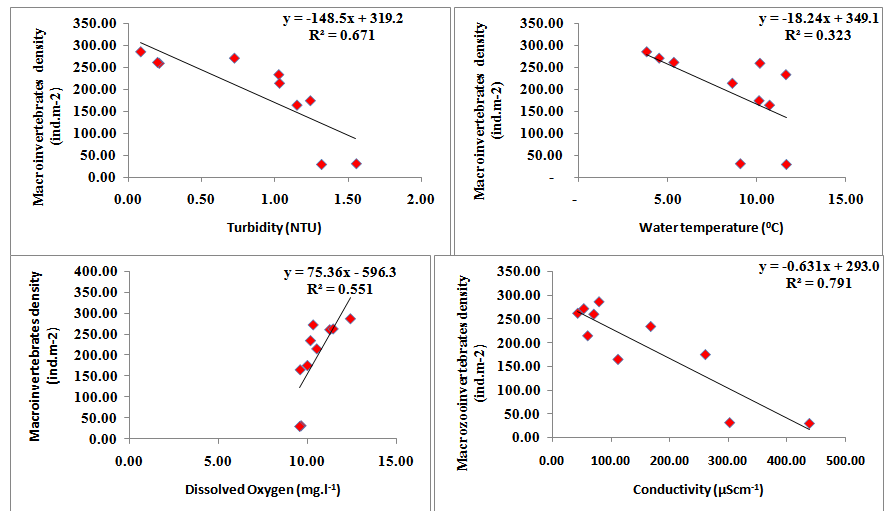
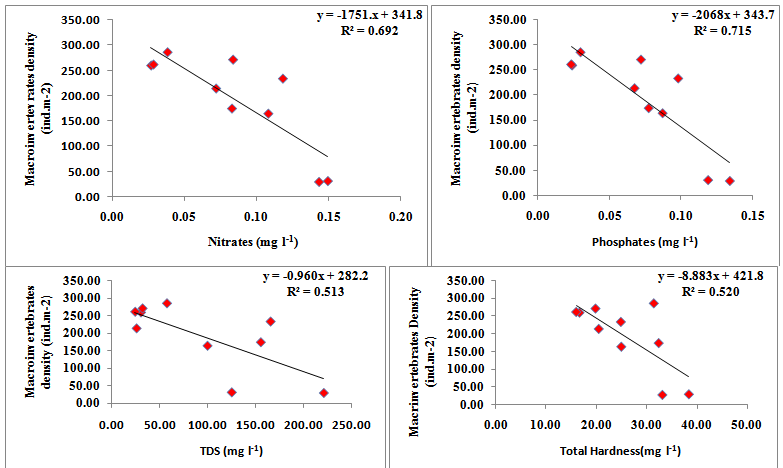
Figure 4 Regression analyses calculated between macroinvertebrates and physicochemical variables of Dodi Tal during the period from November 2014- October 2015.
Shannon Wiener diversity index was found to be maximum (3.66) in winter season and minimum (2.43) in monsoon season in Dodi Tal. Wilhm et al.,30 set diversity index <1 for highly polluted, 1-3 for moderately polluted and >4 for unpolluted water bodies. Bhagat et al.,31 recorded Shannon Wiener diversity index from 1.32-3.32 for macroinvertebrates dwelling the Naukuchiyatal of Kumaon Himalaya. Sharma and Rawat recorded Shannon Wiener index from 3.50-4.41 during their investigation on the Asan wetland, Central Himalayas.
Canonical Correspondance Analysis (CCA) explained the effect of physiochemical parameters on the species of invertebrates. Dissolved oxygen and pH influenced Heptagenia sp., Baetis sp. Mystacides sp., Leptocella sp. Psychomia, Isoperla sp. and Perla sp. at S1. Baetis sp. and Mystacides sp. are the bioindicator of excellent water quality of any freshwater ecosystem.32 These species were found to have positive correlation with axis 1. Temperature, tubidity nitrates, phosphates and chlorides showed negative correlation with Axis 1 and affect the distribution of Enchtreaus sp., Pentaneura sp. and Polycentropus sp. Dissolved oxygen and pH also showed positive correlation with Ephemerella sp., Perla sp. Isoperla sp., Baetis sp. Leptocella sp. and Orthotrichia sp. at S2. Perla sp and Isoperla sp. are the bioindicator of excellent water quality.32 TDS and chlorides influenced the abundance of Polycentopus sp. Enchytreasus sp. and Pentaneura sp. which have negative correlation at Axis 1. Nitrates, phosphates, sulphates and turbidity showed negative correlation with Axis 1 and affect the distribution of Gyraulus sp., Enchytreaus sp. and Pentaneura sp. The distribution and abundance of Ephemerella sp., Perla sp. Isoperla sp., Baetis sp. Setnonema sp. Leptocella sp., Glossosoma and Orthotrichia sp. were influenced significantly by dissolved oxygen and pH at S3 . Chironomus sp. Cybister sp., Halipus sp., Laccophilus sp. Ptilostomis sp. did not show any significant correlation with physico-chemical parameters (Figure 5). Thus, these species are most tolerant in unfavurable conditions of monsoon season. According to Galdean et al.,32 macroinvertebrates groups have been divided into four categories excellent, good, satisfactory and low quality as biological indicator in freshwater ecosystem.33–40
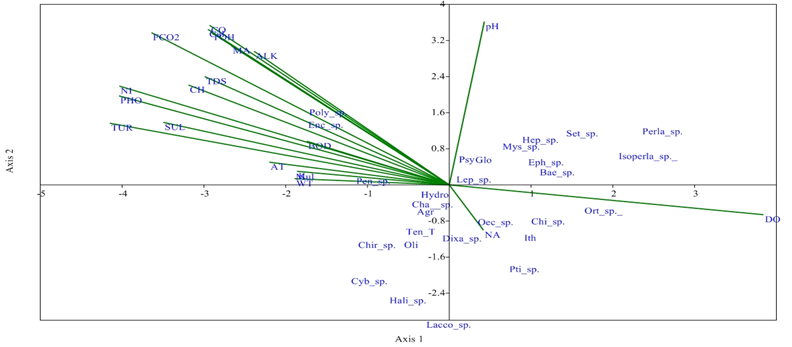
Figure 5 CCA biplot between physiochemical parameters and macro invertebrates species (Physico-chemical parameters: AT, air temperature; WT, water temperature; TUR, turbidity; TDS, total dissolved solid; CO, conductivity; ALK, alkalinity; FCO2, free CO2; DO, dissolved oxygen; BOD, biological oxygen demand; CH, chloride; TOH, total hardness; CA, calcium; MA, magnesium; NI, nitrates; PHO, phosphates; SUL, sulphates; NA, sodium; K, potassium; (Macroinvertebrates species: Hep sp..: Heptagenia sp.; Set sp..: Setnonema sp.; Bae sp: Baetis sp.; Eph sp. :Ephemerella sp.; Perla sp.; Isoperla sp. Poly: Polycentropus sp; Psy: Psychomia; Agr: Agraylea; Hydro: Hydroptila; Ith: Ithytrichia; Or sp: Orthotrichia sp.;Lep sp. : Leptocella sp.; Mys sp: Mystacides sp.; Oec sp. : Oecetis sp.;Oli: Oligostomis reticulata; Pti sp.: Ptilostomis sp.; Glo: Glossosoma; Chi: Chimarra sp Chir sp: Chironomus sp; Dixa sp: Dixa sp.; Pen sp. : Pentaneura sp.; Ten T: Tendipes tentans; Cha sp. :Chaoborus sp.:; Hali sp. : Halipus sp.; Cyb sp.: Cybister sp.; Lac sp.: Laccophilus sp.; Enc sp.: Enchytreaus sp.; Gyr sp.: Gyraulus sp.
The present study revealed that the Heptagenia sp., Ephemerella sp. of Ephemeroptera; Perla sp., Isoperla sp of Plecoptera; Orthotrichia sp., Mystacides sp. of Trichoptera are the potential bioindicator of good health of the high altitude ecosystem of Dodi Tal.41,42 The presence and abundance in high altitude lake Dodi Tal shows excellent water quality at S1 and S2 sites. Maximum density of Oligochaeta was found in summer season and minimum in winter season. Thus, the only species of Oligochaeta (Enchytreaus sp.) can also be treated as potential bioindicator for poor health of the ecosystem. Oligochaeta is a pollution indicator; its presence reflects the poor water quality of the lake at S3 and S4 sites. Thus, the remedial measures (refraining of dumping of waste close to the temple; promotion of public awareness removal of litter) are urgently required at S3 and S4 and during the monsoon season for maintaining the good health of the high altitude wetland, Dodi Tal.43,44
One of the authors (Sushma Singh) is thankful to the University Grants Commission, New Delhi and H.N.B Garhwal University (A Central University) Srinagar - Gharwal, Uttarakhand for providing her fellowship during the period of study.
The authors declare that there are no conflicts of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.