eISSN: 2575-906X


Research Note Volume 2 Issue 1
Department of Materials Sciences, University Centre of Tamanrasset, Algeria
Correspondence: NS Labidi, Department of Materials Sciences, Institute of Science and Technologies, University Centre of Tamanrasset, BP (10034) Sersouf-Tamanrasset-11000, Algeria
Received: January 24, 2018 | Published: February 15, 2018
Citation: Labidi NS. Flotation of barium sulfate contaminants soils. Biodiversity Int J. 2018;2(1):91-93. DOI: 10.15406/bij.2018.02.00049
Application of flotation for the removal of hydrophilic barium sulfate BaSO4 compounds from soil was studied. Micro-flotation tests were conducted on artificially contaminated soil, using a mechanically agitated machine. Flotation parameters tested include collector type and dosage, conditioning time, flotation time and pulp pH. It was found that, with single stage flotation, 85% removal of the contaminant was achieved from soil particles in the range of 80-100μm Flotation was found to have considerable potential for cleaning up contaminated; by combining attrition up to 95% of contaminant barium sulfate was achieved.
Keywords: flotation, soil cleaning, remediation, surfactant, barium
Flotation is, doubtless, the most important process among the various techniques used for the treatment of ore. This general-purpose technique allows the treatment of several complex ores (lead - zinc, copper - zinc), sulphides (copper, lead, and zinc), oxides (hematite, quartz), oxidized minerals (malachite, cerusite) and even non-metal ores (fluorite, phosphate, coal). Due to the flotation, it is possible to concentrate in an economic way poor ores the treatment of which would not be profitable by using other techniques of treatment.1−3
Flotation is a physic-chemical process in which one constituent can selectively be separated from another on the basis of surface properties. This is achieved by controlled additions of chemical reagents at predetermined pH, thereby selectively altering the surface characteristics of barium sulfate BaSO4 compounds contaminated soils. Phase separation is then followed by passing air through reagentized slurry. Air bubbles selectively attach to barium contaminated soil particles and are levitated to the surface in the form of froth. The separation of soil particles contaminated with barium sulfate BaSO4 compounds thus renders the remaining soil clean.4−5 As flotation is a promising method for the removal of hydrophilic minerals compounds from soil, the role of many relevant parameters in soil decontamination is largely unknown. As a result the objective of this study was to test the feasibility of the flotation process for the removal of barium sulfate BaSO4 compounds from contaminated soils and to investigate the effect of basic parameters such as conditioning and flotation time, surfactant dosage, pulp pH.
Soil preparation
The soil used in this study was obtained from a mining company (ENOF, Algeria). The sample was treated with a solution of H2SO4 at 13 %, washed, dried and calcined at 700°C. Detailed of the chemical composition of the sample is given in Table 1. The particle size distribution of the sample used for flotation tests was determined by sieving for 10 min a sample of 500g taken from the ground product for screen analysis (Table 2). The soil sample was then artificially contaminated by mixing it with barium sulfate BaSO4 dissolved in deionized distilled water. This barium sulfate was chosen because of its extremely low solubility and high purity. The low solubility minimizes losses by dissolution during the flotation process, and ensures barium-contaminated soil samples, the desired amounts of soil and barium were first weighed and then a pulp of soil and barium dissolved in deionized distilled water and stirring for 30 minutes before flotation. The mixture of barite and soil after contamination was 1%.6
Elements |
SiO2 |
Al2O3 |
MgO |
Na2O |
Fe2O3 |
CaO |
K2O |
TiO2 |
% |
65.2 |
17.25 |
3.1 |
2.15 |
2.1 |
1.2 |
0.6 |
0.2 |
Table 1 Chemical composition of the soil sample.
Size Range |
Weight (%) |
> 100 μm |
3.55 |
100-80 μm |
19.45 |
80-60 μm |
23.98 |
60-40 μm |
19.96 |
40-20 μm |
18.54 |
20-10 μm |
9.72 |
< 10 μm |
4.8 |
Table 2 Particles size distribution of the soil sample.
Reagents
The barium sulfate contaminant used in this study was obtained from a mining company (mine of Ain-mimmoun Khenchela, Algeria). The purity of the sample was analyzed both by XRD, and by chemical analyses. The purity of the samples was founded to be 99.13%. Detailed of the chemical composition of the sample is given in Table 3. Oleic acid (OA 99%), terpinol (frother) and purchased from ACS. (Algerian Chemical Society) was used throughout the experiments. Freshly prepared oleic acid solutions were used in all the experiments. Dilute solutions of HCl and NaOH were used to adjust the pH of pulp suspension. Tap water was used throughout the study.
% BaSO4 |
%CaO |
%MgO |
% F2O3, |
99.13 |
0.34 |
0.31 |
0.22 |
Table 3 Chemical composition of the barium sample.
Zeta potential measurements
Zeta potential is an indicator of the surface charge of particles, these measurements indicate charge properties of barium and soil particles and in turn can suggest what can adsorb, penetrate, and adhere on particles surface. About 1gm of -100μm for each sample was suspended in 100ml of distilled water. The ionic strength of the suspension was adjusted to 10-3 M by addition of 0.1g of KNO3. The container was inverted 10times and the suspension was allowed to settle for 10 minutes. 60ml of the supernatant, containing the fine particles, was further aged for two hours before electrophoresis mobility measurements were conducted. The pH of the slurry was adjusted using dilute HNO3 and NaOH and was determined before and after the zeta potential measurement using a pH meter and glass electrode jeanway 3300 pH meter. Zeta potential values were then measured and the values reported are the average of ten readings.8−10
Flotation tests
The objective of these experiments was to establish the optimum pH and collector dosage required for separation of substitute barium sulfate from soil matrix. One 1 gram of barium sulfate was conditioned in deionized water for 5 minutes before pH adjustment was carried out. After pH adjustment, required amounts of sodium oleate were added and the sample was further conditioned for 3 minutes. The sample was then quickly transferred to the flotation cell. Three drop of terpinol was added. Flotation was carried out for about 3 minutes. The float and tailings were filtered, dried, weighed and the weight percent recovery was calculated.7
Surface charge measurements
Figure 1 shows the effect of pH on the surface charge of the barite mineral. The point of zero charge (PZC) of barite is around pH 5.2. At pH above 5.2, the surface will be negatively charged, and below pH 5.2 the surface will be positively charged. Since the soil particles are also negatively charged above pH 5.2, surface active reagents can be used which will selectively and preferentially adsorb barium particles and make them hydrophobic.8−10
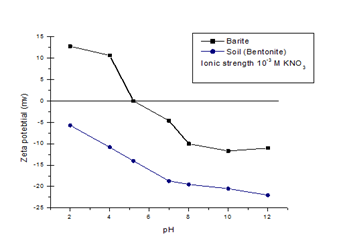
Figure 1 Zeta potential of barite and soil particles as a function of pH. (Ionic strength maintained by addition of 10-3 M KNO3).
Effect of collector concentration
In this series of tests, the concentrations of three reagents (sodium oleate, sodium dodecyl sulfonate and sodium laurate) were evaluated Figure 2. It was found that by increasing the concentration of the collectors, the recovery of barite was increased. It is also evident that the percent removal is strongly dependent on the concentration of the surfactant. An increase in length of the non-polar part of the collector should lead to an increase in its adsorption at interfaces and therefore its separation increased. The length of the hydrocarbon chain of the surfactant was found to affect the flotation of barite.11−14
Effect of pH
Pulp pH plays an important role in the fatty acid flotation of barite. The pH controls the dissociation of fatty acid, the distribution of acid/soap species in solution, and the interfacial properties of the flotation system. The effect of high solids conditioning pH was studied in relation to three reagents (sodium oleate, sodium dodecyl sulfonate and sodium laurate) and barite flotation recovery. The results are presented in Figure 3. At the concentration of collector of 100(mg/l), sodium laurate showed increased recoveries from a value of 15% at pH 2 to 65% at pH 9.5 and decreased at high pH. This could be due to the precipitation of the sodium laurate at high pH. However; Sodium oleate and sodium dodecyl sulfonate(SDS), on the other hand, did not show appreciable change in the recovery values in the pH range 2-12; and the recovery value was maintained at around the 85% level. From these results it can be concluded that sodium oleate and sodium dodecyl sulfonate (SDS) are an effective collector's for recovering barite. On the other hand, sodium dodecanoate is sensitive to the pH of the solution.15
The feasibility of barium elimination from artificially contaminated soil by flotation has been demonstrated. Effects of three types of surfactants (sodium oleate, sodium dodecyl sulfonate and sodium laurate) and their dosage, pulp pH, surface charge measurements were determined. With flotation process over of an 85% barium sulfate removal can be achieved for the 80-100µm soil fraction, using oleic acid as collector at acidic medium pH=4 or alkaline medium pH=9.5 and terpinol as frother.
I gratefully acknowledge the support of this work by the Department of Materials Sciences, Tamanrasset University Centre, Algeria.
Author declares there is no conflict of interest in publishing the article.

©2018 Labidi. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.