eISSN: 2575-906X


Research Article Volume 2 Issue 2
1Laboratório de Conservação e Manejo da Biodiversidade–LabCMBio, Mestrado em Avaliação de Impactos Ambientais, Brazil
2Laboratório de Conservação e Manejo da Biodiversidade–LabCMBio, Curso de Ciências Biológicas, Brazil
3Laboratório de Conservação e Manejo da Biodiversidade–LabCMBio, Mestrado em Avaliação de Impactos Ambientais, Brazil
4Laboratório de Conservação e Manejo da Biodiversidade–LabCMBio, Mestrado em Avaliação de Impactos Ambientais, Brazil
Correspondence: Cristina Vargas Cademartori, Laboratório de Conservação e Manejo da Biodiversidade–LabCMBio, Mestrado em Avaliação de Impactos Ambientais, Universidade La Salle, Unilasalle, Canoas, RS, Brazil
Received: March 28, 2018 | Published: April 26, 2018
Citation: Silva FS, Guimarães LS, Junges SO, et al. Cafeteria experiment with Akodon montensis Thomas, 1913 (Rodentia, Cricetidae, Sigmodontinae) from a remnant of semideciduous seasonal forest in Southern Brazil. Biodiversity Int J. 2018;2(2):204-208. DOI: 10.15406/bij.2018.02.00064
Fruits are an important energy source for a great number of vertebrates. We aim with this study to evaluate the Akodon montensis food selectivity of fruits and animal protein (larvae of Coleoptera and adults of Diplopoda) available monthly in an Atlantic Rainforest remnant in southern Brazil. The individuals were captured monthly from October 2013 to May 2014. The cafeteria experiment was carried out in captivity. Akodon montensis showed preference for small and fleshy fruits and invertebrates.
Keywords: Atlantic forest, food preferences, fruits, invertebrates, small rodents
Rodents represent the largest group of mammals in number of species,1 but the rodent fauna in Brazil is still insufficiently known. Information on the ecology of most species is still scarce,2 especially with regard to their eating habits.3 Most rodents are herbivorous, but some species are generalists, feeding on seeds, fruits and small invertebrates.1 The sigmodontine rodents, although predominantly insectivorous-omnivores, can be more herbivorous or frugivorous.4,5 Akodon montensis Thomas, 1913 is considered a generalist species,1,6,7 which adapts easily to degraded areas, occupying different types of forest formations, opening areas and fields along the Atlantic Forest.6
What an animal consumes is certainly one of the most important aspects of its relationship with the environment. The detailed knowledge of the autoecology of a species allows us to understand the ecological processes in which it is involved, such as its dynamics and its interactions.8 The availability of food resources is one of the most important factors of population maintenance,9 as it influences both the population fluctuations and the survival rate of some species, such as in sigmodontine rodents, whose populations increase in relation to quantity of fruits.10
Neotropical forests, compared to other terrestrial ecosystems, have an exceptionally high number of plants that produce nutrient-rich and palatable fruits that are consumed by some animals because of their proteins and lipids.11 Fruits represent an important energy source for a large number of vertebrates because they are easily found, captured and processed, and the flesh fruit pulp is the primary source of energy for many species of birds, mammals, lizards and even fish.12 Animals defecate, spit, regurgitate, or simply knock fruits away from the parent plant, increasing their chances of survival; therefore, frugivory and dispersion are essential processes for plant populations and for animals.12 Animals are the main seed dispersers in tropical forests and their foraging patterns may have strong effects on plant distribution.13
Although rodents are often considered seed predators,8,14,15 some studies have shown that they can also act as dispersers.16 Thus, they play a crucial role in the development of forests, either by dispersing seeds, serving as a source of food for larger animals or acting as controllers of invertebrate populations.1 However, the degree of frugivory, insectivory and relative importance of each food category are still insufficiently known.15
In southern Brazil, forest fragmentation resulting from increased agricultural frontiers and real estate expansion has restricted forests to hard-to-reach slopes or to conservation areas.17 Considering this scenario, the objective of this study was to evaluate the food selectivity of Akodon montensis from the supply of fruits and animal protein (larvae of Coleoptera and adults of Diplopoda) available monthly in a forest remnant of the Atlantic Forest Domain in southern Brazil. Knowing the food selectivity of small rodents and their response to resource availability contributes to elucidate their influence on the structure and composition of plant communities, as well as to identify potential impacts to these communities.9
Figure 1 Location of the study area in Brazil and the state of Rio Grande do Sul. A, Aerial view of the area where the catches of Akodon montensis were made (source: Google Earth, 2015).

Figure 2 Cafeteria experiment, showing the supply of fruits and animal protein (larvae of Coleoptera and adults of Diplopoda) collected in the study area.
The experiment comprised a total of 18 rodents. The supply of fruits covered 33 plant species (Table 1), with the period from November to January being the most diverse in the supply. Most of the plant species offered were of tree habit (Table 1) and the consumption was similar, suggesting that the easiest way to access these fruits can be given by those that are deposited in the forest floor.
Family |
Species |
Life style |
Average and standard deviation (mm) |
Anacardiaceae |
Schinus terebinthifolius Raddi |
Tree |
3.33±0.60 |
Arecaceae |
Archontophoenix cunninghamii H. Wendl. & Drude |
Palm tree |
12.51±1.37 |
Syagrus romanzoffiana (Cham.) Glassman |
Palm tree |
13.66±0.78 |
|
Cactaceae |
Lepismium cruciforme (Vell.) Miq. |
Epiphyte |
4.28±0.49 |
Rhipsalis teres (Vell.) Steud. |
Epiphyte |
3.63±0.40 |
|
Cannabaceae |
Celtis iguanaea (Jacq.) Sarg. |
Small tree |
10.13±0.42 |
Cucurbitaceae |
Cayaponia martiana Cogn. |
Bindweed |
6.41±0.36 |
Ebenaceae |
Diospyros inconstans Jacq. |
Tree |
19.27±1.28 |
Erythroxylaceae |
Erythroxylum argentinum O.E.Schulz |
Tree |
4.99±0.49 |
Euphorbiaceae |
Sebastiania serrata (Klotzch) Müll.Arg. |
Small tree |
8.27±0.84 |
Lamiaceae |
Vitex megapotamica (Spreng.) Moldenke |
Tree |
13.79±1.26 |
Moraceae |
Ficus cestrifolia Schott |
Tree |
10.11±0.17 |
Ficus microcarpa Vahl. |
Tree |
5.08±0.36 |
|
Sorocea bonplandii (Baill.) W.C. Burger. Lanjouw & Boer |
Small tree |
12.08±0.42 |
|
Myrtaceae |
Myrciaria cuspidata O.Berg |
Tree |
6.77±0.34 |
Campomanesia xanthocarpa O.Berg |
Tree |
13.92±2.04 |
|
Eugenia hiemalis Cambess. |
Tree |
7.01±0.02 |
|
Eugenia involucrata DC. |
Tree |
21.1±1.56 |
|
Eugenia uniflora L. |
Tree |
8.73±0.27 |
|
Nyctaginaceae |
Guapira opposita (Vell.) Reitz |
Tree |
9.35±0.91 |
Phytolaccaceae |
Rivina humilis L. |
Erva |
3.43±0.88 |
Primulaceae |
Myrsine umbellata Mart. |
Tree |
6.24±0.06 |
Rubiaceae |
Psychotria brachyceras Müll. Arg. |
Bush |
8.9±0.73 |
Psychotria carthagenensis Jacq. |
Bush |
4.52±0.39 |
|
Salicaceae |
Banara parviflora (A. Gray) Benth. |
Tree |
5.85±1.03 |
Sapindaceae |
Allophylus edulis (A.St.-Hil., Cambess. & A. Juss.) Radlk. |
Tree |
7.87±0.14 |
Cupania vernalis Cambess. |
Tree |
7.1±1.16 |
|
Solanaceae |
Cestrum strigillatum Ruiz & Pav. |
Bush |
7.18±0.82 |
Solanum sanctaecatharinae Dunal |
Tree |
10.13±0.18 |
|
Thymelaeaceae |
Daphnopsis racemosa Griseb. |
Small tree |
4.46±0.65 |
Urticaceae |
Coussapoa microcarpa (Shott) Rizzini |
Tree |
6.66±0.88 |
Urera bacífera (L.) Gaudich. |
Bush |
4.3±0.51 |
|
Vitaceae |
Cissus striata Ruiz & Pav. |
Bindweed |
7.04±0.80 |
Table 1 List of plant species, life form, mean and standard deviation of the diameter of the fruits offered21.
Most of the studies on the rodent diet cite them as generalist species, without many specializations,1,5,15 which seems to agree with A. montensis, because 87%, that is, more than half of the fruit sample was totally consumed or in part, although some fruits were avoided at some time (Figure 3). Vieira et al.15 also obtained similar results in a study in which most of the fruits offered to A. montensis were consumed. In a test carried out in captivity with eight rodent species, Vieira et al.5 arrived at the same findings.
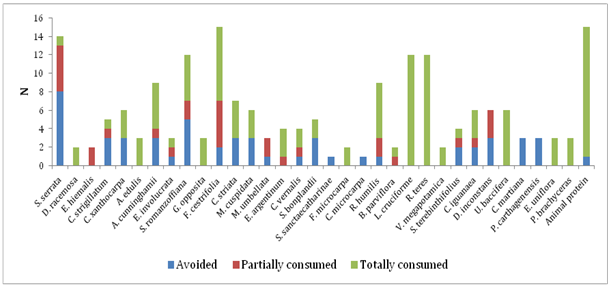
Figure 3 Consumption of fruits and animal protein (larvae of Coleoptera and adults of Diplopoda) by Akodon montensis in a remnant of Semideciduous Seasonal Forest of southern Brazil.
The fruits of Allophylus edulis (A.St.-Hil., Cambess. & A. Juss.) Radlk., Banara parviflora (A. Gray) Benth., Celtis iguanaea (Jacq.) Sarg., Daphnopsis racemosa Griseb., Erythroxylum argentinum O.E. Schulz., Eugenia hiemalis Cambess., Lepismium cruciforme (Vell.) Miq., Rhipsalis teres (Vell.) Steud., Rivina humilis L., Urera bacífera (L.) Gaudich., Vitex megapotamica (Spreng.) Moldenke., Ficus microcarpa and Guapira opposita were totally or partially consumed, never avoided (Figure 3), comprising 39% of the vegetal species offered, which may indicate a certain preference in relation to the species whose fruits were at some time avoided. The fruits of L. cruciforme and Rhipsalis teres, in turn, were consumed integrally in all the times they were offered (40% of the rodents consumed these fruits in different periods of sampling), suggesting a preference for fleshy fruits, since both are of the berry type. Ratnaweera & Wijesinghe9 also revealed a tendency of rats to consume fruits with fleshy pulp, thin bark and easily digestible. Rhipsalis teres is an epiphytic, rupicolous or ground plant and L. cruciforme is epiphytic or rupicolous.21 The fact that they are terrestrial and rupicolous suggests that the acquisition of fruits by rodents of terrestrial habit can be due to direct access to the plant, which would be more difficult if they exhibited an exclusively epiphytic habit, since these fruits are very small and therefore difficult to be found in the substrate.
The fruits of Cayaponia martiana Cogn., Psychotria carthagenensis Jacq., Solanum sanctaecatharinae Dunal. and Coussapoa microcarpa were avoided 100% of the time (Figure 3). Aversion to plants or plant parts in particular may result from olfactory and gustatory responses, presence of spines, hard or dense bark, among other structural features, and also as a result of a harmful reaction to toxic secondary metabolites.9
Among all the plant species offered, two are exotic, Archontophoenix cunninghamii H. Wendl. & Drude and Ficus microcarpa, and both were consumed. Therefore, rodents can act as dispersers of these species if they do not damage the seeds. Research in coniferous forest in the northern US indicated a higher consumption of native species seeds, but also consumption of exotic species by granivorous rodents, concluding that the role of small mammals as agents of biotic resistance to invasion of plants is complex.15 Animal protein (invertebrates) was offered to all individuals and avoided by only one, demonstrating an evident preference for this source of food (Figure 3). Studies on diet and frugivory also showed a higher consumption of invertebrates by A. montensis.8
Regarding the diameter of the fruits, the majority of the supply included fruits of smaller size. However, considering the variation in the diameter of the sample, from 3 to 21 mm, fruits between 3 and 9 mm appear to have been the most accepted (Figure 4), indicating a preference for smaller fruits and with greater availability. In the Atlantic Forest, between 60 and 90% of the species are zoocoric,24 and several shrub and tree plants produce small fruits (up to 5 cm in diameter), with a high concentration of volatile aromatic compounds that serve as attractions for the mastofauna.11 These results differ from those presented by Vieira et al.,15 in which no differences were found in the size of the fruits consumed by the studied rodents, including A. montensis.
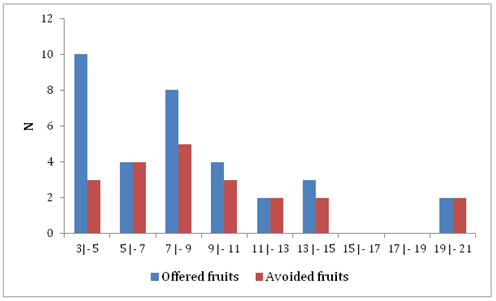
Figure 4 Frequency distribution of fruit size (diameter in mm) offered and avoided by Akodon montensis in a remnant of Semideciduous Seasonal Forest of southern Brazil.
Jordano et al.14 state that the regeneration process does not depend exclusively on specialized frugivores, but on a large number of generalist species, with a diet generally based on fruits and insects. Knowing that A. montensis is abundant in the area and adapts to degraded environments, it may contribute to the recovery of forests such as Morro do Coco, by dispersing pioneering seeds that give conditions to the colonization of other species. Therefore, they should help to maintain the populations and communities of plants and, consequently, in the recovery and conservation of ecosystems.
The results suggest that Akodon montensis exhibits food selectivity, since while animal protein (larvae of Coleoptera and adults of Diplopoda) was consumed by almost all individuals, some fruits were consumed while others were avoided. Although small rodents are more broadly considered generalists, A. montensis has shown a preference for small, fleshy fruits and animal protein.
The authors declare there is no conflict of interest.

©2018 Silva, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.