eISSN: 2575-906X


Research Article Volume 2 Issue 2
Department of Environmental Sciences, HNB Garhwal University, India
Correspondence: Vijayta Tiwari, Department of Environmental Sciences, HNB Garhwal University (A Central University), Srinagar-Garhwal, Uttarakhand, India
Received: December 23, 2017 | Published: March 2, 2018
Citation: Sharma RC, Tiwari V. Phytoplankton diversity in relation to physico-chemical environmental variables of Nachiketa Tal, Garhwal Himalaya. Biodiversity Int J. 2018;2(2):102?110. DOI: 10.15406/bij.2018.02.00052
The study deals with the assessment of physico-chemical parameters and phytoplankton diversity of Nachiketa Tal. The lake was monitored for one annual cycle (May 2014-April 2015). A total of 71 taxa of phytoplankton belonging to 57 genera were recorded. Phytoplankton in the lake are represented by six major families, Bacillariophyceae (22), Chlorophyceae (37), Cyanophyceae (6), Xanthopyceae (2), Dinophyceae (2) and Euglenophyceae (2). The overall phytoplankton density was found to be abundant (1401±325.60 ind. l-1) in winter season, declined in monsoon season (457.50±17.83 ind. l-1) but the population of Cyanophyceae and Euglenophyceae was found to be high (174.33±37.09 ind. l-1) and (24.33±5.89 ind. l-1) respectively in summer season. Results showed that the increases in turbidity, TDS in monsoon season are the driving factors for decreasing the phytoplankton density in the lake. Pearson’s correlation and CCA calculated between environmental variables and phytoplankton diversity showed that the distribution of phytoplankton in the lake is dependent on the variabilities of physico-chemical variables.
Keywords: phytoplankton, himalaya, lake, physico-chemical
Lakes are the important natural resources present on the Earth. These are the type of productive ecosystem important for any geographical region, as they play significant role in its ecological stability.1 The Nachiketa Tal is an important lake of Garhwal Himalaya. This lake is surrounded by dense forest of Querecus, Abies, Rhododendron and Myrica, etc. and receives its water from the watershed. The variations and distribution of phytoplankton in freshwater lake depend on its physiochemical features.2 Phytoplanktons are the primary producer and play a vital role in food chain of aquatic ecosystem.3,4 Due to this reason, phytoplanktons are usually used as an ecological indicator to assess the ecological health and the stress effects of chemical contaminants on aquatic ecosystems and they are also necessary to sustain a healthy aquatic ecosystem.5‒8 Dynamics in the phytoplankton biomass are the result of the complex interaction of physical, chemical and biological processes. The availability of nutrients influences the diversity of the phytoplankton. From the past few decades, there is much interest to study various factors influencing the development of phytoplankton in relation to the physico-chemical attributes.9‒12 Changes in the phytoplankton community of different types of lakes also assessed the trophic status and environmental quality of the lakes. Algae (Phytoplankton) are photoautotrophic organisms having chlorophyll ‘a’ and unicellular reproductive structures. It is highly diverse group, which are important for aquatic habitats.13
A preliminary study on the primary productivity of Nachiketa Tal has been done by Singh et al.14 A factual report of the water quality of the lake is also available.15 So the present study was carried out in order to determine the composition, density and diversity of Phytoplankton of Nachiketa Tal and the influence of physico-chemical variables on them.
Nachiketa Tal is located between latitude N 30°38.666’ N and longitude E 078°28.362’ E at an altitude of 2,453m above m.s.l. in Garhwal Himalaya. The area of Nachiketa Tal is 0.49 ha. It is elliptical in shape, measuring about 132 m in length and 58 m width (Figure 1). The lake remains occasionally covered with thin sheet of ice during the winter months of January and February. The lake depth varies from season to season and attains its maximum depth during rainy season and has no noticeable inlet as well as outlet. During the major part of the year, Nachiketa Tal remains shallow. The runoff from the forest deposited sediments in the lake bed. Monthly sampling was undertaken at 08:00 to 10:00 hrs during the one annual cycle (May 2014 to April 2015).
Four sampling sites (S1, S2, S3, S4) were identified for covering the entire ecosystem for collecting data on the physico-chemical parameters and phytoplankton community of the Nachiketa Tal. For physico-chemical parameters, five replicates of samples from each sampling site were taken for each parameter and their mean value was computed. Water temperature was recorded with the help of the Centigrade (0-110°C) thermometer; electrical conductivity and pH of the sample were measured with the help of the Toshcon Multiparameter Analyser. Nitrates, phosphates, sulphates were determined by using Spectrophotometer (Model –UV-VIS Systronics 117 series). Dissolved Oxygen, total hardness, BOD, alkalinity, calcium, magnesium etc. were measured followed the method outlined in Wetzel and Likens,16 APHA.17
For phytoplankton, from each sampling site, five liter of sample was passed through silk plankton net of mesh size 20 µm and was fixed through 4% formalin solution into sample bottles. Enumeration of density of phytoplankton (ind. l-1) was done by using Sedgwick Rafter counting cell. The identification of phytoplankton was done by using the Olympus CH 20i Microscope and was identified with the help of Ward & Whipple,18 Bellinger & Sigee19 and Munshi et al.20 The Pearson’s correlation coefficient was used to determine the relationship among the various physico-chemical parameters and different phytoplankton. The correlation coefficient was performed using SPSS version 16.0. CCA was performed using P Aleonotological Statistics (PAST) Software version 2.10.
Physico-chemical environmental variables
The high altitude lakes are sensitive indicators of environmental changes. The water qualities of such lakes are greatly influenced by the variations in parameters like temperature and pH.21 The physico-chemical parameters were determined in order to observe their influence on phytoplankton density (Table 1-4). Air temperature was found maximum (22.13±1.53°C) at S4 in summer season and minimum (10.43±2.58°C) at S2 in winter season. Water temperature was found to be maximum (20.77±1.72°C) in summer season at S4 and minimum (8.7±1.9°C) in winter at S2. Electrical conductivity was highest (69.9±2.4µScm-1) in monsoon season at S2 and lowest (34.57±2.99µScm-1) in winter at S1. pH is an important parameter and has a significant role in the biological process of all aquatic organisms.22 pH was found maximum(7.280.07) at S4 in winter season and lowest (6.59±0.01) was recorded in monsoon season at S2. TDS in Nachiketa Tal was found maximum (36.75±0.35 mg.l-1) at S2 in monsoon season and minimum (16.3±1.27mg. l-1) at S3 in spring season. Turbidity was found maximum in monsoon (7.97±0.97 NTU) at S2 and minimum in spring (2.96±1.15 NTU) at S4. Maximum TDS and turbidity in monsoon season is due to the rain which transports soil and other organic matter from the watershed to the lake. The availability of dissolved oxygen is one of the most critical factors for the survival of the aquatic organisms.23 DO was found maximum (8.57±0.45 mg.l-1) at winter season at S4 and minimum (6.5±0.71 mg.l-1) at S1 in monsoon season. Higher dissolved oxygen in winter season is also reported by Garg et al.24 BOD was found to be maximum (1.5±0.14 mg.l-1) in monsoon season at S4 and minimum (0.57±0.12 mg.l-1) in winter season at S3. Similar finding was recorded by Bordoloi & Baruah.25 Free CO2 was found maximum (2.38±0.62 mg.l-1) and minimum (1.01±0.19 mg.l-1) at S2 in monsoon and spring season respectively. Higher free CO2 was also recorded in monsoon season.25 Maximum water temperature in the Nachiketa Tal was recorded high in the month of summer followed by months of monsoon. Raised temperature in the monsoon season could increase the microbial decomposition of the organic matter carried out by the rain in to the lake ecosystem which in turn reduce the DO level into the water body.25‒28 Due to the microbial decomposition, there is an increased demand of DO, resulting in the high BOD value in the monsoon season.29 High microbial activities in the monsoon season also raise the level of CO2 into the water, which is also one of the reasons of maximum CO2 level in monsoon season. Maximum free CO2 decreases the pH level in monsoon season.30 Total alkalinity was found maximum (82.73±3.68 mg.l-1) and minimum (53.50±11.43 mg.l-1) in summer season and winter season respectively at S1. Similar trend was also reported by Fathi and Flower.31 Calcium was found maximum (8.28±2.02 mg.l-1) in summer season at S1 and minimum (4.01±1.13 mg.l-1) in monsoon at S2. Magnesium was found to be maximum (3.82±0.11 mg.l-1) at S3 in summer season and minimum in monsoon season at S1, S3 and in autumn season at S3. Chlorides were found maximum (5.68±0.8 mg.l-1) in monsoon at S2 and minimum (2.98±0.2 mg.l-1) in autumn season at S3.Total hardness was found to be maximum (34.67±6.11 mg.l-1) in summer season at S1 and minimum (21±1.41 mg.l-1) in monsoon at S2 and S3. Nitrates were found to be maximum (0.372±0 mg.l-1) in monsoon season at S2 and minimum (0.256±0.01 mg.l-1) in winter season at S4. Similar finding was reported by Panigrahi et al.32 Phosphates were found maximum (0.086±0.01mg.l-1) in monsoon season at S1 and minimum (0.057±0 mg.l-1) in spring season at S3. Sulphates were found to be maximum (7.85±0.28 mg.l-1) in monsoon at site S3 and minimum (5.38±0.39 mg.l-1) in S3 at autumn.
Parameters |
Summer |
Monsoon |
Autumn |
Winter |
Spring |
Air temp (0C) |
21.77±1.53 |
21.65±1.2 |
19.15±0.49 |
11±2.71 |
13±7.78 |
Water temp. (0C) |
20.5±1.87 |
19.75±1.06 |
16.9±0.99 |
9.17±1.8 |
11.85±7 |
Conductivity (µScm-1) |
58.47±5.03 |
68.45±2.76 |
56.4±2.55 |
34.57±2.99 |
48.5±2.83 |
Alkalinity (mg.l-1) |
82.73±3.68 |
78.2±2.83 |
67.9±10.47 |
53.5±11.43 |
63.25±6.72 |
TDS (mg l-1) |
27.67±6.71 |
36.25±1.77 |
17.2±1.98 |
16.7±0.44 |
16.35±1.63 |
Free CO2 (mg l-1) |
1.58±0.77 |
2.24±0.68 |
1.06±0.12 |
1.76±0.88 |
1.06±0 |
Dissolved oxygen (mg.l-1) |
7±0.4 |
6.5±0.71 |
6.7±0.42 |
8.4±0.72 |
8.3±0.71 |
BOD (mg.l-1) |
1.3±0.26 |
1.35±0.21 |
1.05±0.21 |
0.7±0.1 |
1±0.28 |
pH |
6.76±0.04 |
6.73±0.06 |
6.86±0.06 |
7.27±0.06 |
7.11±0.36 |
Turbidity (NTU) |
5.47±0.87 |
7.78±0.91 |
5.15±1.27 |
3.2±0.06 |
3.12±1.04 |
Chlorides (mg l-1) |
3.6±0.87 |
5.4±0.4 |
3.55±0.2 |
3.22±0.91 |
3.98±0.4 |
Hardness(mg l-1) |
34.67±6.11 |
22±2.83 |
23±1.41 |
26.73±1.1 |
25±1.41 |
Calcium(mg l-1) |
8.28±2.02 |
5.21±0.57 |
5.61±0 |
6.84±0.74 |
6.01±0.57 |
Magnesium (mg l-1) |
3.4±0.48 |
2.18±0.34 |
2.19±0.34 |
2.35±0.18 |
2.43±0 |
Nitrates (mg l-1) |
0.322±0.01 |
0.355±0.01 |
0.334±0.03 |
0.271±0.03 |
0.287±0.03 |
Phosphates (mg l-1) |
0.064±0.01 |
0.086±0.01 |
0.078±0 |
0.066±0.01 |
0.06±0 |
Sulphates (mg.l-1) |
6.02±0.8 |
7.72±0.57 |
5.38±0.59 |
6.48±0.62 |
5.73±0.66 |
Table 1 Seasonal variation in physico-chemical parameters at sampling stations S1 of Nachiketa Tal, Garhwal Himalaya, Uttarakhand during the period from May 2014 to April 2015
Parameters |
Summer |
Monsoon |
Autumn |
Winter |
Spring |
Air temp (°C) |
21.37±0.85 |
21.25±1.06 |
18.6±0.57 |
10.43±2.58 |
12.6±7.92 |
Water temp. (0C) |
19.67±1.04 |
19.55±0.64 |
16.55±0.78 |
8.7±1.9 |
11.35±6.86 |
Conductivity (µScm-1) |
59.37±6 |
69.9±2.4 |
58.1±3.25 |
35.87±2.95 |
49.5±3.25 |
Alkalinity (mg.l-1) |
81.6±5.72 |
80±3.54 |
68.15±10.82 |
53.77±11.23 |
62.5±3.54 |
TDS (mg l-1) |
29.6±6.16 |
36.75±0.35 |
17.85±2.33 |
17.37±0.72 |
17.75±2.62 |
Free CO2 (mg l-1) |
1.67±0.69 |
2.38±0.62 |
1.14±0.12 |
1.61±0.67 |
1.01±0.19 |
Dissolved oxygen (mg.l-1) |
6.83±0.35 |
6.65±0.49 |
6.9±0.14 |
8.43±0.4 |
8.3±0.42 |
BOD (mg.l-1) |
1.33±0.31 |
1.35±0.21 |
0.8±0.14 |
0.63±0.15 |
0.85±0.21 |
pH |
6.62±0.07 |
6.59±0.01 |
6.8±0.07 |
7.13±0.06 |
7.06±0.37 |
Turbidity (NTU) |
5.69±0.95 |
7.97±0.97 |
5.24±1.36 |
3.35±0.18 |
3.34±1.11 |
Chlorides (mg l-1) |
3.88±0.43 |
5.68±0.8 |
3.41±0 |
3.12±0.85 |
3.83±0.6 |
Hardness(mg l-1) |
33.33±5.03 |
21±1.41 |
22.4±2.26 |
26.83±1.44 |
25±1.41 |
Calcium(mg l-1) |
7.48±1.85 |
4.01±1.13 |
4.81±1.13 |
6.41±1.61 |
5.61±1.14 |
Magnesium (mg l-1) |
3.56±0.74 |
2.67±1.03 |
2.52±0.14 |
2.63±0.69 |
2.67±0.35 |
Nitrates (mg l-1) |
0.335±0.01 |
0.372±0 |
0.355±0.05 |
0.268±0.02 |
0.296±0.04 |
Phosphates (mg l-1) |
0.065±0 |
0.084±0.01 |
0.076±0 |
0.065±0.01 |
0.06±0 |
Sulphates (mg.l-1) |
6.29±0.68 |
7.74±0.3 |
5.69±0.33 |
6.66±0.58 |
5.95±0.75 |
Table 2 Seasonal variation in physico-chemical parameters at sampling stations S2 of Nachiketa Tal, Garhwal Himalaya, Uttarakhand during the period from May 2014 to April 2015
Parameters |
Summer |
Monsoon |
Autumn |
Winter |
Spring |
Air temp (°C) |
21.87±1.52 |
21.2±1.41 |
19±0.71 |
10.93±2.58 |
12.75±7.85 |
Water temp. (0C) |
20.67±1.46 |
19.75±0.64 |
16.9±0.57 |
8.9±1.85 |
11.65±6.72 |
Conductivity (µScm-1) |
57.87±4.71 |
67.2±3.25 |
57.05±3.46 |
34.63±3.36 |
48.35±2.62 |
Alkalinity (mg.l-1) |
80.4±7.29 |
78.35±2.62 |
67.7±10.61 |
53.63±11.83 |
64±5.66 |
TDS (mg l-1) |
27.57±6.19 |
36.35±1.63 |
17.05±1.63 |
16.87±0.55 |
16.3±1.27 |
Free CO2 (mg l-1) |
1.55±0.79 |
2.2±0.62 |
0.97±0.12 |
1.53±0.57 |
1.06±0.12 |
Dissolved oxygen (mg.l-1) |
7.07±0.31 |
6.7±0.71 |
6.85±0.21 |
8.37±0.85 |
8.1±0.14 |
BOD (mg.l-1) |
1.13±0.12 |
1.2±0.28 |
0.9±0.14 |
0.57±0.12 |
0.8±0.28 |
pH |
6.71±0.11 |
6.69±0.05 |
6.85±0.07 |
7.17±0.15 |
7.05±0.35 |
Turbidity (NTU) |
5.28±0.93 |
7.75±0.77 |
5.16±1.35 |
3.17±0.05 |
3.03±1.1 |
Chlorides (mg l-1) |
3.6±0.71 |
5.25±0.6 |
2.98±0.2 |
3.22±0.91 |
3.98±0.4 |
Hardness(mg l-1) |
32±4 |
21±1.41 |
22±2.83 |
26.67±1.15 |
25.2±1.7 |
Calcium(mg l-1) |
6.52±1.45 |
4.81±0 |
5.21±0.57 |
6.68±0.46 |
5.93±1.13 |
Magnesium (mg l-1) |
3.82±0.11 |
2.18±0.34 |
2.18±0.34 |
2.43±0 |
2.53±0.27 |
Nitrates (mg l-1) |
0.324±0.01 |
0.36±0.01 |
0.334±0.01 |
0.263±0.02 |
0.291±0.04 |
Phosphates (mg l-1) |
0.065±0 |
0.084±0 |
0.077±0.01 |
0.062±0 |
0.057±0 |
Sulphates (mg.l-1) |
6.2±0.92 |
7.85±0.28 |
5.38±0.39 |
6.51±0.55 |
5.82±0.7 |
Table 3 Seasonal variation in physico-chemical parameters at sampling stations S3 of Nachiketa Tal, Garhwal Himalaya, Uttarakhand during the period from May 2014 to April 2015
Parameters |
Summer |
Monsoon |
Autumn |
Winter |
Spring |
Air temp (°C) |
22.13±1.53 |
21.8±1.41 |
19.3±0.71 |
11.13±2.52 |
13.15±7.99 |
Water temp. (°C) |
20.77±1.72 |
19.7±0.99 |
17.25±0.64 |
9.27±1.87 |
11.75±6.72 |
Conductivity (µScm-1) |
58.37±5.15 |
67.35±4.03 |
56.2±3.39 |
34.7±3.46 |
48.4±3.11 |
Alkalinity (mg.l-1) |
80.57±4.38 |
78.5±2.83 |
67.6±10.75 |
54.27±11.87 |
65±4.24 |
TDS (mg l-1) |
26.97±6.47 |
36.7±1.84 |
17.2±1.7 |
16.93±0.51 |
16.35±1.2 |
Free CO2 (mg l-1) |
1.55±0.79 |
2.24±0.56 |
1.06±0 |
1.53±0.57 |
1.06±0.12 |
Dissolved oxygen (mg.l-1) |
6.97±0.4 |
6.7±0.42 |
7±0.28 |
8.57±0.45 |
8.3±0.14 |
BOD (mg.l-1) |
1.4±0.17 |
1.5±0.14 |
1±0.28 |
0.6±0.1 |
0.95±0.21 |
pH |
6.7±0.03 |
6.69±0.04 |
6.75±0.07 |
7.28±0.07 |
7.09±0.37 |
Turbidity (NTU) |
5.53±1 |
7.77±0.83 |
5.15±1.34 |
3.19±0.05 |
2.96±1.15 |
Chlorides (mg l-1) |
3.5±0.71 |
5.11±1.2 |
3.41±0 |
3.31±0.82 |
3.55±0.2 |
Hardness(mg l-1) |
34±6 |
22±0 |
22±2.83 |
26.67±1.15 |
25±1.41 |
Calcium(mg l-1) |
8.02±1.61 |
4.97±0.23 |
4.41±1.7 |
6.41±0 |
6.01±0.57 |
Magnesium (mg l-1) |
3.4±0.48 |
2.33±0.14 |
2.67±0.34 |
2.59±0.28 |
2.43±0 |
Nitrates (mg l-1) |
0.334±0 |
0.357±0 |
0.323±0 |
0.256±0.01 |
0.287±0.04 |
Phosphates (mg l-1) |
0.064±0.01 |
0.086±0 |
0.08±0 |
0.066±0 |
0.061±0 |
Sulphates (mg.l-1) |
6.24±0.96 |
7.84±0.55 |
5.49±0.33 |
6.5±0.65 |
5.8±0.82 |
Table 4 Seasonal variation in physico-chemical parameters at sampling stations S4 of Nachiketa Tal, Garhwal Himalaya, Uttarakhand during the period from May 2014 to April 2015
The water system in Nachiketa Tal was mainly influenced by rainfall. So, it is estimated that any variation, whether seasonal or monthly in physico-chemical variables of Nachiketa Tal may be influenced by climatic factors and the characteristics of its catchment. The high seasonal variations in electrical conductivity in Nachiketa Tal in monsoon season were may be because of the rains which export the ions from the catchment area to the lake ecosystem. Similar trend was reported by Burna et al.33 The nutrient like phosphates, nitrates and sulphates increases in the monsoon because the rains brought these nutrients from the catchment. The decrease of nutrients during the winter season may be due to the phytoplankton population.
Plankton community
Physico-chemical parameters of any water body alone do not provide an ideal picture of the ecological condition of the water body. The biotic community of any water body is outcome of interaction between the chemical, physical and geo-morphological characteristics of any water body.34,35 The study of physico-chemical parameter is important to know their impact on phytoplankton and vice-versa. A total of 71 taxa belonging to 57 genera were recorded in the lake Nachiketa Tal. The phytoplankton in the lake is represented by six major families, Bacillariophyceae (22), Chlorophyceae (37), Cyanophyceae (6), Xanthopyceae (2), Dinophyceae (2) and Euglenophyceae (2). The percentage compositions (Figure 2) of these six families were Bacillariophyceae (31%), Chlorophyceae (52%), Cyanophyceae (8%), Euglenophyceae (3%), Xanthophyceae (3%) and Dinophyceae (3%). Overall, maximum density (1501 ind. l-1) of phytoplankton was observed in January at S1 and minimum (443 ind.l-1) phytoplankton density was observed in July at site S3. Seasonally, the maximum density 1401±325.60 ind. l-1 of phytoplankton was observed in winter season at S1 and minimum (457.50±17.83 ind. l-1) in monsoon season at S2 (Figure 3). Dissolved oxygen is produced by phytoplankton biomass due to their photosynthetic activity. Therefore, dissolved oxygen was found higher, where phytoplankton count was high.12 Density of Cyanophyceae and Euglenophyceae was found high in summer season. The present study also recorded higher dissolved oxygen and the phytoplankton during winter season and lowest during monsoon season. During the monsoon season there was an increased amount of turbidity in Nachiketa Tal due to runoff from the watershed, which transports soil and other plant and animal debris into the lake. During the period of high turbidity in the outflow zone, the nutrient concentration increased and the transparency decreased. It was found that the phytoplankton density decreased during this period. Thus, the light is an important factor for the growth of phytoplankton through the process of photosynthesis.13,36 The Chlorophyceae was recorded as the dominant group in the lake ecosystem of Nachiketa Tal followed by Bacillariophyceae and Cyanophyceae. Similar findings were reported by Tyagi & Malik.37
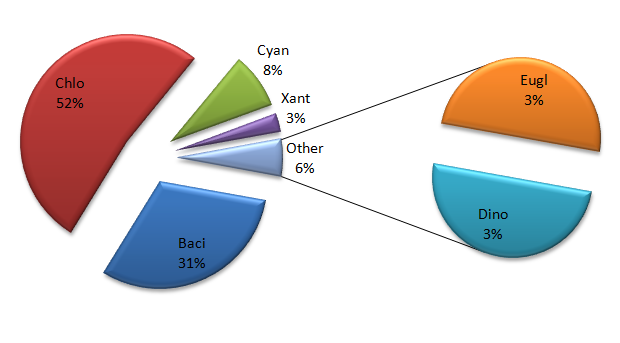
Figure 2 Percentage composition of phytoplankton in Nachiketa Tal (Chlo, Chlorophyceae; Baci, Bacillariophyceae; Cyan, Cyanophyceae; Xant, Xanthophyceae; Eugl, Euglenophyceae; Dino, Dinophyceae).
Chlorophyceae
Chlorophyceae has been recorded as the dominant component of phytoplankton followed by Bacillariophyceae, Cyanophyceae, Xanthophyceae, Dinophyceae and Euglenophyceae respectively. Hinder et al.38 also recorded Chlorophyceae as a dominant groups over the other groups in Jöri lakes, Swiss Alps; Bhat et al.39 also recorded Chlorophyceae as a dominant group in Bhoj wetland, a Ramsar Site. Sharma40 also reported Chlorophyceae as dominant group in Loktak Lake. The dominating species of this group were Staurastrum manipurense, Scendesmus quardicauda, Spirogyra sp., Ankistrodermus falcatus, and Chlorella vulgaris. A total of 37 species were recorded from the Nachiketa Tal. The maximum density of Chlorophyceae (710 ind. l-1) at S1 in January and lowest (211 ind. l-1) in July at S4. Seasonally, the population of Chlorophyceae was recorded maximum (663.67±158.15 ind. l-1) in winter season and minimum (221.5±86.97 ind. l-1) in monsoon season. Maximum density of Chlorophyceae was recorded in winter season by Tiwari & Chauhan.41
Bacillariophyceae
The second most abundant group recorded during the present study was Bacillariophyceae. The dominant species were Cymbella sp., Navicula sp., Nitzschia sp, and Synedra sp. similar results were reported by Kumar et al.42 in the ponds of Badrinath. The maximum (703 ind. l-1) density was recorded at S1 in January and lowest (116 ind. l-1) in August at S3. Seasonally, the population of Bacillariophyceae was recorded maximum (629.33±126.09 ind. l-1) in winter season and minimum (118.5±41.72ind. l-1) in monsoon season.
Cyanophyceae
The third group dwelling Nachiketa Tal was Cyanophyceae. The dominating species were Chroococcus sp. Gloeocapsa sp., Oscillatoria sp., Microcystis sp. Density of Cyanophyceae was found maximum (184 ind. l-1) in June and minimum (11 ind. l-1) in December at S3. Seasonally, it was found maximum (174.33±37.09 ind. l-1) during summer season and minimum (12.67±8.08 ind. l-1) during winter season. Similar findings were reported by Tiwari & Chauhan41 and Rosińska et al.43 Increased temperature and long photoperiod in summer supports the increased Chlorophyceae diversity.39 After the summer season, second highest density of Cyanophyceae was seen in monsoon season (121.5±20.5 ind. l-1) in Nachiketa Tal. Jarousha44 reported that the high concentration of nitrates in monsoon season attributed higher member of Cyanophyceae. The concentration of nitrates was found maximum in monsoon season in the lake.
Two genera of each family of Xanthophyceae, Dinophyceae and Euglenophyceae were encountered in Nachiketa Tal. The maximum density of Xanthophyceae (27 ind. l-1) in December at S4. Density of Dinophyceae was found maximum (52 ind. l-1) during November and December at S1 and at S4 in November. Euglenophyceae were found maximum (31 ind. l-1) in June at site S1. Seasonally, the maximum density of Xanthophyceae (21±7 ind. l-1) and Dinophyceae (43.33±1 ind. l-1) was found to be in winter season at S1. Euglenophyceae were found maximum (24.33±5.89 ind. l-1) in summer season at site S1. The Shannon-Wiener diversity index was recorded maximum (3.950) in winter at S2 and minimum (3.750) in monsoon at S4 and the Jaccard evenness was recorded maximum (0.067) in autumn at S1 and S3 and minimum (0.057) in summer at S1, S2 and S4 during the present study. The pollution indicating taxa like Navicula, Oscillatoria, Nitzschia, Euglena, Cymbella were encountered in almost all the four sites with varied numbers in Nachiketa Tal.42,45 Numbers of these taxa were less at S3 in comparison to other sites.
Statistical treatment of data
Pearson’s correlation coefficient calculated between various physico-chemicals attributes and density of phytoplankton dwelling Nachiketa Tal have been presented in Table 5. Water temperature and pH have negative correlation (r=-989, p<0.05). TDS has positive correlation with the water temperature (r=0.719, p<0.01) and electrical conductivity (r=0.771, p<0.01). Abundance of Bacillariophyceae, Chlorophyceae, Xanthophyceae, Dinophyceae is negatively correlated with water temperature (r=-0.898, p<0.01); (r=-845, p<0.01); (r=-875, p<0.01) and (r=-0.761, p<0.01) respectively. However, the abundance of Cyanophyceae and Euglenophyceae were positively correlated with water temperature (r=0.881, p<0.01) and (r=0.619, p<0.01) respectively. Turbidity is negatively correlated with Bacillariophyceae (r=-0.882, p<0.01), Cholorophyceae (r=-0.802, p<0.01) and Xanthophyceae (r=-0.721, p<0.01). However, it is positively correlated with Cyanophyceae (r=0.625, p<0.05).Turbidity may be the one of the reasons of lower phytoplankton density in the monsoon season. DO was positively correlated with Bacillariophyceae (r=0.919, p<0.01), Cholorophyceae (r=0.766, p<0.01), Xanthophyceae (r=-0.883, p<0.01) and Dinophyceae (r=0.751,p<0.01). However, it was negatively correlated with Cyanophyceae (r=-668, p<0.05). Cyanophyceae showed positive correlation with nitrates (r=-0.686, p<0.05). Phosphates are negatively correlated with the density of Bacillariophyceae (r=-0.686, p<0.05).
AT |
WT |
EC |
ALK |
TDS |
FCO2 |
DO |
BOD |
pH |
TUB |
Cl |
TH |
Ca |
Mg |
NO3 |
PO4 |
SO4 |
BAC |
CHL |
CYN |
XAN |
DIN |
EUG |
|
AT |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WT |
.994** |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EC |
.853** |
.873** |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ALK |
.788** |
.830** |
.837** |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TDS |
.690* |
.719** |
.771** |
.694* |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FCO2 |
0.306 |
0.292 |
0.283 |
0.119 |
.626* |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DO |
-.865** |
-.869** |
-.898** |
-.844** |
-.658* |
-0.101 |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BOD |
.871** |
.903** |
.905** |
.915** |
.839** |
0.389 |
-.792** |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
pH |
-.985** |
-.989** |
-.886** |
-.828** |
-.718** |
-0.29 |
.868** |
-.915** |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TUB |
.807** |
.807** |
.887** |
.766** |
.847** |
0.477 |
-.858** |
.849** |
-.839** |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cl |
0.447 |
0.435 |
.586* |
0.46 |
0.538 |
0.196 |
-0.524 |
0.476 |
-0.451 |
.590* |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
TH |
0.134 |
0.188 |
-0.051 |
0.274 |
0.196 |
0.123 |
0.02 |
0.228 |
-0.096 |
-0.104 |
-0.464 |
1 |
|
|
|
|
|
|
|
|
|
|
|
Ca |
-0.083 |
-0.03 |
-0.22 |
0.088 |
0.067 |
0.098 |
0.194 |
0.015 |
0.127 |
-0.228 |
-0.546 |
.944** |
1 |
|
|
|
|
|
|
|
|
|
|
Mg |
0.436 |
0.479 |
0.22 |
0.497 |
0.35 |
0.133 |
-0.252 |
0.503 |
-0.416 |
0.109 |
-0.234 |
.863** |
.647* |
1 |
|
|
|
|
|
|
|
|
|
NO3 |
.867** |
.863** |
.901** |
.699* |
.661* |
0.343 |
-.812** |
.835** |
-.910** |
.878** |
0.501 |
-0.201 |
-0.384 |
0.124 |
1 |
|
|
|
|
|
|
|
|
PO4 |
0.542 |
0.503 |
.644* |
0.29 |
0.561 |
0.477 |
-.652* |
0.41 |
-0.536 |
.778** |
0.523 |
-0.486 |
-0.507 |
-0.346 |
.690* |
1 |
|
|
|
|
|
|
|
SO4 |
0.119 |
0.115 |
0.236 |
0.141 |
0.54 |
.656* |
-0.161 |
0.191 |
-0.081 |
0.412 |
.617* |
-0.12 |
-0.032 |
-0.227 |
0.135 |
0.495 |
1 |
|
|
|
|
|
|
BAC |
-.892** |
-.898** |
-.978** |
-.815** |
-.716** |
-0.301 |
.919** |
-.882** |
.915** |
-.882** |
-.579* |
0.102 |
0.298 |
-0.221 |
-.935** |
-.686* |
-0.214 |
1 |
|
|
|
|
|
CHL |
-.809** |
-.845** |
-.925** |
-.894** |
-.809** |
-0.348 |
.766** |
-.965** |
.855** |
-.802** |
-.615* |
-0.109 |
0.091 |
-0.391 |
-.805** |
-0.414 |
-0.28 |
.893** |
1 |
|
|
|
|
CYN |
.840** |
.881** |
.741** |
.852** |
.696* |
0.245 |
-.668* |
.909** |
-.856** |
.625* |
0.28 |
0.496 |
0.264 |
.739** |
.686* |
0.114 |
0.023 |
-.722** |
-.846** |
1 |
|
|
|
XAN |
-.882** |
-.875** |
-.886** |
-.758** |
-0.526 |
-0.149 |
.883** |
-.774** |
.875** |
-.721** |
-0.564 |
0.115 |
0.329 |
-0.238 |
-.834** |
-.585* |
-0.114 |
.943** |
.811** |
-.666* |
1 |
|
|
DIN |
-.715** |
-.761** |
-.844** |
-.800** |
-0.478 |
0.121 |
.751** |
-.769** |
.781** |
-.611* |
-0.373 |
-0.001 |
0.148 |
-0.229 |
-.763** |
-0.318 |
0.107 |
.811** |
.802** |
-.714** |
.778** |
1 |
|
EUG |
.613* |
.619* |
0.433 |
0.561 |
.711** |
.648* |
-0.402 |
.692* |
-.589* |
0.559 |
0.256 |
0.508 |
0.318 |
.686* |
0.445 |
0.147 |
0.35 |
-0.451 |
-.587* |
.743** |
-0.351 |
-0.129 |
1 |
Table 5 Correlation between the physico-chemical attributes of the Nachiketa Tal, Garhwal Himalayas, during the period from May 2014 to April 2015
**; Correlation is significant at the 0.01 level (2-tailed).
*. Correlation is significant at the 0.05 level (2-tailed).
Abbreviations: AT, air temp.; WT, water temp.; EC, electrical conductivity; ALK, alkalinity; TDS, total dissolve soilds; FCO2, free carbon dioxide; DO, dissolve oxygen; BOD, bio-chemical oxygen demand; TUB, turbidity; Cl, chloride; TH, hardness; Ca, calcium; Mg, magnesium; NO3, nitrates;PO4, phosphates; SO4, sulphates; BAC, bacillariophyceae; CHL, Chlorophyceae; CYN, cyanophyceae; XAN, xanthophyceae; DIN, dinophyceae; EUG, Euglenophyceae.
Canonical correspondence analysis
Canonical Correspondence Analysis (CCA) method was used to found the relationship between the physico-chemical variables and phytoplankton. It is a direct gradient analysis technique,46 it extracts synthetic gradient from the biotic and environmental matrices and quantitatively represented by arrows in graphical biplots.47 In the present study, the eigenvalues and the percentage of variance for each site were found to be high for axis 1 than axis 2. CCA was drawn between the 17 physico-chemical parameters and 42, 37, 41, 40 dominant phytoplankton species from S1, S2, S3 and S4respectively.
At the sampling site S1, (Figure 4). The Eigen values were 0.124 and 0.042 for axis 1 and axis 2 respectively. The axis 1 shows 56.86 % correlation and axis 2 shows 19.26 % correlation with the physico-chemical parameters and dominant phytoplankton species. The species like Microspora sp., Staurastrum manipurense, Pinnularia sp., Synedra ulna, Chlorella vulgaris, Cymbella affinis, Frustulia sp., Scenedesmus quardicauda are strongly related with axis 1. Nitzschia sp., Navicula radiosa, Navicula sp., Ankistrodesmus falcatus, Cymbella affinis, Frustulia sp., Diatoma sp., Monoraphidium sp., Synedra ulna are positively related with the dissolved oxygen and pH. Water temperature, BOD, alkalinity, TDS, electrical conductivity, and nitrates were found to have in positive correlation with Gloeocapsa sp. and Chroococcus sp.
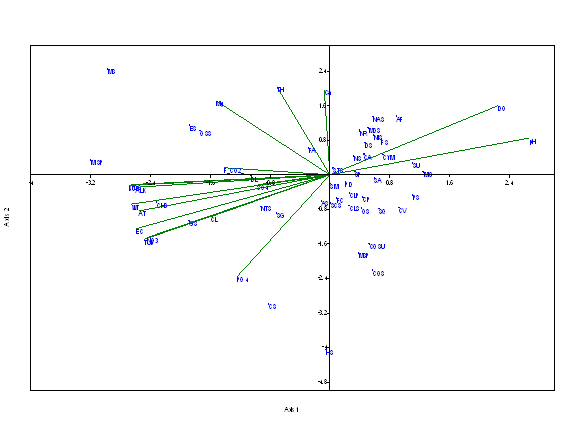
Figure 4 CCA biplot between physico-chemical parameters and species of phytoplankton at S1 (Dominant phytoplankton species: CLP, Closterium pseudodianae; CLS, Closterium sp. COS, Cosmarium sp.; COSU, Cosmarium subtumidium; DL, Dimorphococcus lunatus; HS, Hormidium sp.; MS, Mesotaenium sp.; MIS, Microspora sp.; MOS, Monoraphidium sp.; MSP, Mougeotia sp.; OS, Odegonium sp.; CA, Cymbella aequalis; CYM, Cymbella affinis; ES, Euglena sp.;OSS, Oscillatoria sp.; NTS, Nostoc sp.; MISP, Microcystis sp.; GS, Gloeocapsa sp.; CHS, Chroococcus sp.; SM, Staurastrum manipurense; STS, Staurastrum spp.; SP, Spirogyra sp.; SG, Selenastrum gracile; SCS, Scenedesmus sp.; SQ, Scenedesmus quadricauda; SA, Scenedesmus accuminatus; PD, Pediastrum duplex;DS, Diatoma sp.; FA, Fragilaria arcus; FC, Fragilaria capucina; FS, Frustulia sp.;NR, Navicula radiosa;NS, Navicula sp.; NAS, Navicula subtilissima; NIS, Nitzschia sp.; PS, Pinnularia sp.; SU, Synedra ulna; AF, Ankistrodesmus falcatus; AS, Ankistrodesmus spiralis;CS, Chlamydomonas spp.; CV, Chlorella vulgaris; CP, Closterium parvulum
Eigen values of 0.122 explained 53.58% correlation with axis 1 for the S2 (Figure 5) Eigen value (0.041) explained 18% correlation with axis 2. Axis 1 was strongly related with the species like Cymbella affinis, Synedra ulna, Pinnularia sp., Microspora sp., Diatoma sp., Navicula subtilissima, Nitzschia sp., Gonatozygon sp., Closterium parvulum. Whereas, Oscillatoria sp., Ankistrodesmus falcatus, Microcystis sp., Fragilaria arcus, Gloeocapsa sp. were positively correlated with the axis 2. Dissolved oxygen and pH were positively correlated with the genera of Bacillariophyceae (Cymbella affinis, Synedra ulna, Navicula subtilissima, Nitzschia sp.). Bacillariophyceae was reported high in the winter season, when dissolved oxygen and pH were found maximum. Although the seasonal variations in water temperature, electrical conductivity, TDS, free CO2, turbidity, nitrates negatively influence these species.
Eigen value (0.101) explained 46.68% for axis 1 and Eigen value (0.040) explained 18.77 % for axis 2 at S3 (Figure 6). Alkalinity, TDS, water temperature, nitrates, turbidity, BOD are positively related to axis 1 and positively related with Cyanophyceae member like Gloeocapsa sp. and Chroococcus sp., which increases in summer to monsoon season, when there is an increase in water temperature, Nitrates were found in maximum concentration in monsoon season due to surface runoff in Nachiketa Tal. The dissolved oxygen and pH are negatively related with the axis 1. Phosphates were positively correlated with the Scendesmus sp. The Eigen value (0.113) explained by 50.66% of correlation at S4 (Figure 7). Eigen value (0.046) for axis 2 explained 20.83 % correlation. Dissolved oxygen and pH were strongly related with the axis 1. Nitzschia sp., Frustulia sp., Navicula radiosa, Microspora sp., Pinnularia sp., Synedra ulna and Ankistrodesmus falcatus are positively correlated with dissolved oxygen and pH but the parameters like water temperature, turbidity, electrical conductivity, BOD and nitrates influences these species.
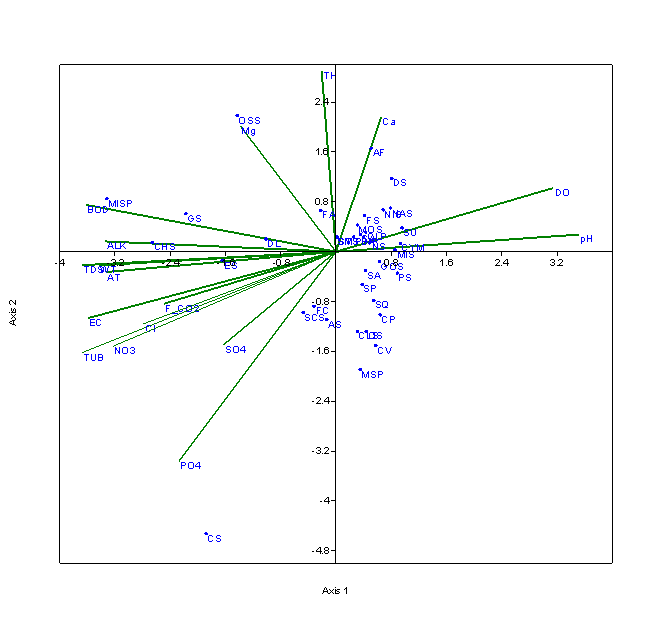
Figure 5 CCA biplot between physico-chemical parameters and species of phytoplankton at S2 (Dominant phytoplankton species: CLP, Closterium pseudodianae; CLS, Closterium sp.; DL, Dimorphococcus lunatus; GOS, Gonatozygon sp.; HS, Hormidium sp.; MIS, Microspora sp.; MOS, Monoraphidium sp.; MSP, Mougeotia sp.; OS, Odegonium sp.; CA, Cymbella aequalis; CYM, Cymbella affinis;ES, Euglena sp.; OSS, Oscillatoria sp.; MISP, Microcystis sp.; GS, Gloeocapsa sp.; CHS, Chroococcus sp.; SM, Staurastrum manipurense; STS, Staurastrum spp.; SP, Spirogyra sp.; SCS, Scenedesmus sp.; SQ, Scenedesmus quadricauda; SA, Scenedesmus accuminatus; PD, Pediastrum duplex; DS, Diatoma sp.; FA, Fragilaria arcus; FC, Fragilaria capucina; FS, Frustulia sp.; NR, Navicula radiosa; NS, Navicula sp.; NAS, Navicula subtilissima;NIS, Nitzschia sp.; PS, Pinnularia sp.; SU, Synedra ulna; AF, Ankistrodesmus falcatus; AS, Ankistrodesmus spiralis; CS, Chlamydomonas spp.; CV, Chlorella vulgaris; CP, Closterium parvulum
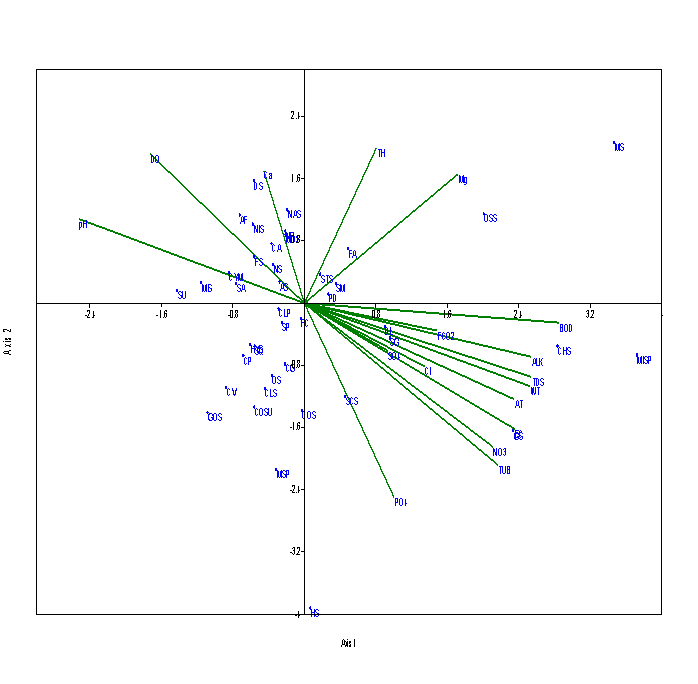
Figure 6 CCA biplot between physico-chemical parameters and species of phytoplankton at S3 (Dominant phytoplankton species: CLP, Closterium pseudodianae; CLS, Closterium sp.; COS, Cosmarium sp.; COSU, Cosmarium subtumidium; DL, Dimorphococcus lunatus; HS, Hormidium sp.; MS, Mesotaenium sp.; MIS, Microspora sp.; MOS, Monoraphidium sp.; MSP, Mougeotia sp.; OS, Odegonium sp.; CA, Cymbella aequalis; CYM, Cymbella affinis; ES, Euglena sp.; OSS, Oscillatoria sp.; MISP, Microcystis sp.; GS, Gloeocapsa sp.; CHS, Chroococcus sp.; SM, Staurastrum manipurense; STS, Staurastrum spp.; SP, Spirogyra sp.; SG, Selenastrum gracile; SCS, Scenedesmus sp.; SQ, Scenedesmus quadricauda; SA, Scenedesmus accuminatus; PD, Pediastrum duplex; DS, Diatoma sp.; FA, Fragilaria arcus; FC, Fragilaria capucina; FS, Frustulia sp.; NR, Navicula radiosa; NS, Navicula sp.; NAS, Navicula subtilissima;NIS, Nitzschia sp.; SU, Synedra ulna; AF, Ankistrodesmus falcatus; AS, Ankistrodesmus spiralis; CV, Chlorella vulgaris; CP, Closterium parvulum; CG, Cosmarium granatum;GOS, Gonatozygon sp.; HYS, Hydrodiction sp.
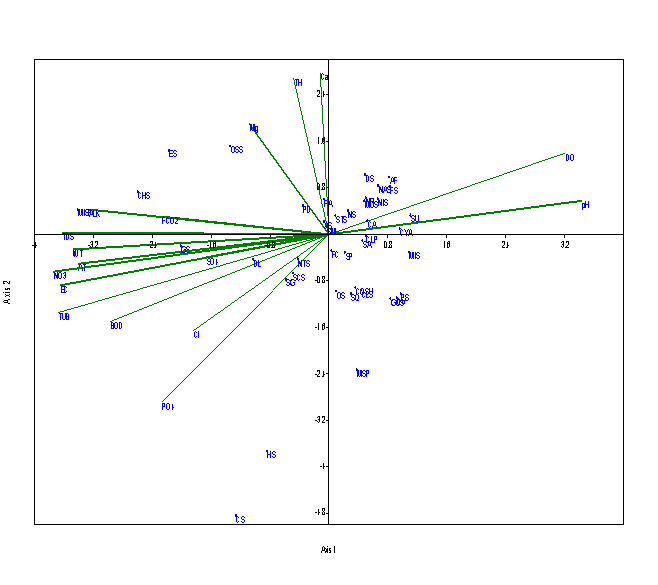
Figure 7 CCA biplot between physico-chemical parameters and species of phytoplankton at S4 (Dominant phytoplankton species: CLP, Closterium pseudodianae; CLS, Closterium sp.; COS, Cosmarium sp.; COSU, Cosmarium subtumidium; DL, Dimorphococcus lunatus; HS, Hormidium sp.; MIS, Microspora sp.; MOS, Monoraphidium sp.; MSP, Mougeotia sp.; OS, Odegonium sp.; CA, Cymbella aequalis; CYM, Cymbella affinis; ES, Euglena sp.; OSS, Oscillatoria sp.; NTS, Nostoc sp.; MISP, Microcystis sp.; GS, Gloeocapsa sp., CHS, Chroococcus sp.; SM, Staurastrum manipurense; STS, Staurastrum spp.; SP, Spirogyra sp.; SG, Selenastrum gracile; SCS, Scenedesmus sp.; SQ, Scenedesmus quadricauda; SA, Scenedesmus accuminatus; PD, Pediastrum duplex;DS, Diatoma sp.; FA, Fragilaria arcus; FC, Fragilaria capucina; FS, Frustulia sp.; NR, Navicula radiosa; NS, Navicula sp.; NAS, Navicula subtilissima; NIS, Nitzschia sp.; PS, Pinnularia sp.; SU, Synedra ulna; AF, Ankistrodesmus falcatus; AS, Ankistrodesmus spiralis; CS, Chlamydomonas spp.; CV, Chlorella vulgaris; CP, Closterium parvulum; GOS, Gonatozygon sp.
Phytoplanktonic density and diversity have significant correlation with the physico-chemical variables in Nachiketa Tal. The growth of phytoplankton was directly or indirectly dependent on the environmental variables of the Lacustrine ecosystem. The environmental attributes like dissolved oxygen, pH, turbidity, TDS, BOD and nutrients influenced the population of the phytoplankton dwelling in the Nachiketa Tal. Navicula, Cymbella, Nitzschia, Oscillitoria, Nostoc, Microcystis etc. were recorded indicators of the pollution under the present study. It is evident from the results that the lake is progressing from the Oligotrphic to the Mesotrophic state.
One of the authors (Vijayta Tiwari) is thankful to the University Grant Commission and H.N.B Garhwal University (A Central University) for providing Central University fellowship for undertaking the present work.
There is no conflict of interest to declare regarding the publication of this paper.

©2018 Sharma, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.