eISSN: 2575-906X


Research Article Volume 2 Issue 1
1FES-Zaragoza, UNAM, Mexico
2Geographical Information Systems Laboratory, Institute of Biology, National Autonomous University of Mexico, Mexico
3Los Tuxtlas Tropical Biology Station, Institute of Biology, National Autonomous University of Mexico, Mexico
Correspondence: Rosamond Coates, Los Tuxtlas Tropical Biology Station, Institute of Biology, National Autonomous University of Mexico, Carr. Catemaco-Montep
Received: November 28, 2017 | Published: January 17, 2018
Citation: Gutiérrez-Granados G, León-Villegas RI, Rodríguez-Moreno A, et al. Loss of connectivity in a highly fragmented tropical landscape: use of ecological processes as functional indicators of biodiversity decline. Biodiversity Int J. 2018;2(1):28-35. DOI: 10.15406/bij.2018.02.00039
Loss of functional connectivity in tropical forests worldwide has been attributed to the increasing socio-economic pressures on land use and has caused severe forest fragmentation as well as loss of ecological processes. In this paper, we analyze the functional connectivity among different-sized patches through several processes/species indicators from the Los Tuxtlas region. We establish three minimal patch sizes based on a group of different indicators considering 1000 m as the minimum distance between patches. For this analysis, we used the Integral Index of Connectivity (IIC) and Probability of Connectivity (PC) indexes and three subcategories (intra, flux and conn) in order to prioritize forest remnants. Using each of these criteria, we ranked the selected forest patches to devise a functional connectivity strategy for the entire area. The Los Tuxtlas region is functionally disconnected, but has two large enough patches, which allow the maintenance of processes/species. Wasp and bird diversity persist in smaller fragments. While other processes like pollination, tree recruitment and plant demography are more sensitive to fragmentation. Processes based on community structure and genetic diversity are present only in large-sized fragments. We affirmed that the reestablishment of connectivity would be possible if smaller fragments were incorporated into management programs in order to obtain a managed landscape that allows long-term conservation of tropical rainforests.
Keywords: Ecological function conservation, fragmentation indicators, los tuxtlas, tropical rainforest, conservation, patches connectivity
Landscape modification has been increasing in the past few decades. In particular, the biodiversity of tropical rainforests has been threatened by an intensive loss of habitat and changes in land use policies.1−3 These processes modify the spatial pattern of the remaining forests increasing for example patch isolation,3,4 potentially affecting wildlife through changes in ecological processes such as pollination, seed dispersal, seed predation and herbivory.5,6 In addition, the consequences of this habitat modification have driven wildlife toward population decline and, in some cases, local species extirpations.7,8
In order to respond to this biodiversity crisis, landscape management strategies are required to minimize population and species extinction by reducing conservation conflicts.8 In this regard, landscape conformation can exert a strong influence on extinction probabilities, therefore, not only the spatial distribution of patches across a landscape is important, but connectivity is also scale-dependent since each species interacts with landscape patterns and spatial distribution in different ways.9 Therefore, knowing the optimal size of patches that are able to maintain wildlife is of the highest conservation concern.9 In addition, all other patch characteristics must be considered in order to allow wildlife movement between isolated patches10 to ensure long-term conservation.11,12 Species movements throughout the landscape playan important role in recovery from disturbances by facilitating dispersal and recolonization.13 Nonetheless, large-scale land use intensification is reducing and fragmenting natural areas, consequently decreasing the quality of functional connections among habitats. Thus, enhancing landscape connectivity is a major concern influencing management strategies in nature conservation.14,15
It has been recommended that improvement of indicators of landscape connectivity must contain two components. Firstly, the monitoring of movement of species should be considered. However, over large spatial and temporal scales this is costly and challenging. As a result, it is necessary and increasingly common to use indicators of connectivity, i.e., connectivity metrics that can incorporate available knowledge on species dispersal but do not require the specific gathering of empirical movement data for each monitoring or planning application,16,17 Secondly, a list of species is required and their use of individual patches in a region, or sets of patches in each local area within a region.13 These two components give decision-support guidelines that could be applied by conservation practitioners in order to select the adequate indicators for the identification of general landscape management strategies.
Landscape alterations seem to be the common denominator for tropical rainforests around the world.18−20 However due to ecological processes and alteration effects works at different scales it is almost always a challenge to document and devising landscape solutions.21 Thus, the integration of several studies focusing on ecological processes will help to generate indicators that allow an integrated landscape management is currently more important in the context of landscape sustainability.22,23
A worthy example of this worldwide situation is the Los Tuxtlas region in the state of Veracruz, Mexico. This region has suffered from intensive deforestation over at least six decades20,24,25 leaving an anthropogenic landscape with intermingled natural forested areas of different sizes.26 Here, the fragmentation effects on several species of wildlife have been studied (see bibliography in table 1) and in particular, there is a study on the influence of indigenous management on general connectivity.27 Yet no connectivity attempts have been documented in order to improve long-term indicators that favor conservation using a wide range of processes/species. Therefore, in this paper we analyzed the functional connectivity among different sized patches for several wildlife species or processes from the Los Tuxtlas region in order to obtain ecological indicators that show the effects at different landscape scales. Then, we discussed the global implications of considering functional connectivity in fragmented tropical forests and finally, we suggest a type of management tool for tropical sites with similar landscape characteristics.
Study area
We conducted the spatial analysis in the Los Tuxtlas region in the southeast portion of the state of Veracruz, Mexico (18°8′18. 45″N 94° 37′. 95. 22″W; Figure 1).The climate is warm and humid with an annual precipitation of 4000-4900 mm, with a marked dry season from March to May. The original vegetation of the Los Tuxtlas region is classified as high evergreen tropical rainforest.28,29 The area is considered a biodiversity hotspot, as it is the northernmost limit of this type of vegetation in the Neotropics and is under intense anthropogenic pressure (for further details see 30).
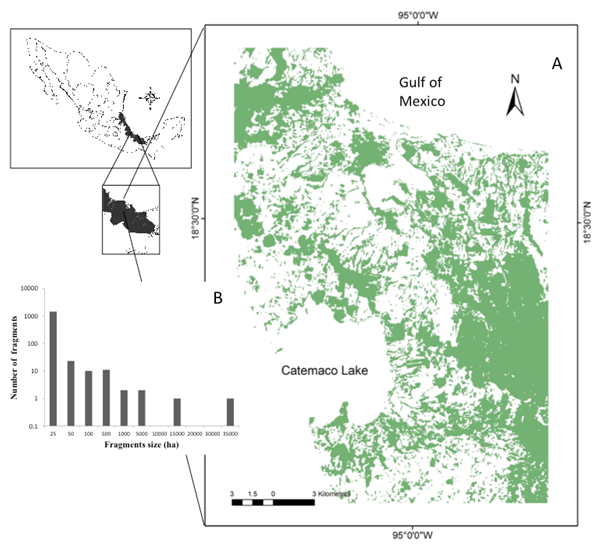
Figure 1 Natural vegetation areas considered in functional connectivity analysis (A) and number of < 50 ha fragments (B) in Los Tuxtlas region.
Landscape analysis
We used a Land sat 5 TM (path/Row 23/47) satellite image from April 8, 2011 to detect, at the landscape level, the remnant number of natural vegetation patches with the minimum size to maintain processes/species. The image has a spatial resolution of 30 m. We applied a non-supervised classification and the resulting image was reclassified with three clump-eliminate processes to preserve the topographic forms. The result was representative of the land cover of the terrain. We only took into account the patches with a minimum size of four pixels (3600 m2). For landscape analysis, we used the central area (ca. 55,800 ha, hereafter termed as the Los Tuxtlas Region) of the Los Tuxtlas Biosphere Reserve. This area was selected because it contains the highest number of representative patches of tropical rain forest within the Biosphere Reserve.
We performed a functional connectivity analysis31 in order to identify the most critical landscape elements for the maintenance of wildlife, taking into account the overall landscape connectivity in the Los Tuxtlas region. Since the area is heavily fragmented, we established three minimal patch sizes to determine the functional connectivity. First, we analyzed the connectivity scenario (CS1) between the lesser represented >150 ha patches. The second connectivity scenario (CS2) considers patches of >50 ha. Finally, we established a third connectivity scenario (CS3) using all fragments >25 ha, which are the ones most commonly represented in the Los Tuxtlas region. To set the minimum distance between patches we used the dispersal capacity of a selected species groups from different taxa and analysis level (Table 1). Using this data, we established 1000m as the largest distance that any of the species could cross through an unsuitable matrix (cattle pastures and croplands) in order to reach suitable habitat. We used the Integral Index of Connectivity (IIC) and Probability of Connectivity (PC) indexes to estimate the functional connectivity between patches, and we used three subcategories (intra, flux and conn) in order to prioritize forest remnants.11,31 Using each of these criteria, we ranked the selected forest patches in the Los Tuxtlas region in order to devise a functional connectivity strategy for the entire area.
Taxon |
Analysis Level |
Effect |
Fragment Size Threshold (ha) |
Connectivity Scenario |
Reference |
Heliconia uxpanapensis |
Genetic diversity |
NE |
nd |
3 |
57 |
Ateles geoffroyi |
- |
nd |
1 |
58 |
|
Alouatta palliata |
- |
nd |
1 |
58 |
|
Seedremoval |
NE |
5 |
3 |
59 |
|
Wasps (Ichneumonidae) |
- |
10 |
3 |
60 |
|
Lianas |
+ |
0.5-76 |
2 |
61 |
|
Insectivorous Bird diversity |
- |
19 |
3 |
34 |
|
pollinators |
- |
34 |
2 |
38 |
|
pollinators |
- |
34 |
2 |
62 |
|
Poulsenia armata |
Recruitment |
- |
40 |
2 |
39 |
Poulsenia armata |
Demographic |
- |
40 |
2 |
40 |
Poulsenia armata |
Seed predation |
NE |
40 |
2 |
63 |
Dung beetles |
- |
60 |
1 |
64 |
|
Astrocaryum mexicanum |
- |
75 |
1 |
65 |
|
Alouatta palliata |
- |
75 |
1 |
66 |
|
Primary Vegetation species |
- |
75 |
1 |
67 |
|
Secondary Vegetation species |
+ |
75 |
1 |
67 |
|
Dieffenbachia seguine |
Genetic diversity |
- |
100 |
1 |
68 |
Non flying mammals |
- |
100 |
1 |
69 |
|
Frugivorous Birds |
- |
100 |
1 |
69 |
|
Frugivorous Bats |
- |
100 |
1 |
69 |
|
Medium-sized mammals |
- |
125 |
1 |
32 |
|
Panthera onca |
- |
15000 |
4 |
Pers. comm. (RC) |
Table 1 Minimal fragment size and connectivity scenario of selected processes/species from different taxonomical groups and analysis level in Los Tuxtlas Mexico.
NE: No effect; nd: No data; -: Negative effect; +: Positive effect.
Species information
We conducted a literature survey on previous research carried out on the effects of fragmentation or patch size for wildlife in the Los Tuxtlas region, and then evaluated and filtered all the results. Additionally, we used some of our own non-flying mammal data as an in situ fragmentation example.32 Using these data, we developed a score that allowed us to rank species according to their minimal size patch use to maintain presence/functionality in the Los Tuxtlas region. Processes/species were classified as habitat (1) highly suitable, (2) suitable, (3) less suitable and (4) not suitable depending on previously documented sensibility to fragmentation. Then, each species was assigned to one of the scenarios established in the connectivity assessment.
The Los Tuxtlas region shows severe loss in its functional connectivity. The area has a total of 1,478 fragments, of which 98% are less than 150 ha. Therefore, none of the processes/species used as indicators to evaluate the connectivity would be able to find a completely suitable habitat in this highly fragmented landscape. However, the most conservative scenario, CS1, held only two large enough patches to attain biodiversity, but nearly 70% of processes/species lost their functionality under the CS2 and CS3 assessment. The jaguar (Panteraonca) was the only species classified in the category 4 due to its large home range, and consequently has little opportunity to attain an ecologically functional presence.
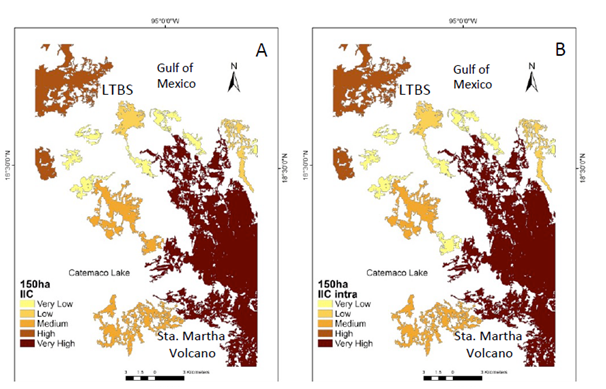
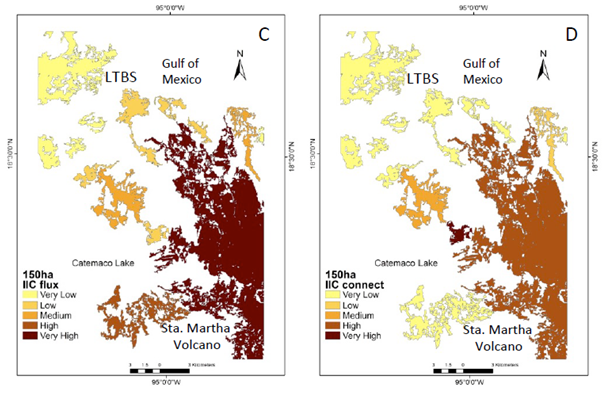
Figure 2 Natural vegetation patches > 150 ha (CS1) important for the maintenance of functional connectivity. (A) Patches are ranked according to their values of IIC. (B) The sub-categories IICintra. (C) IICflux (D) IICconnect.
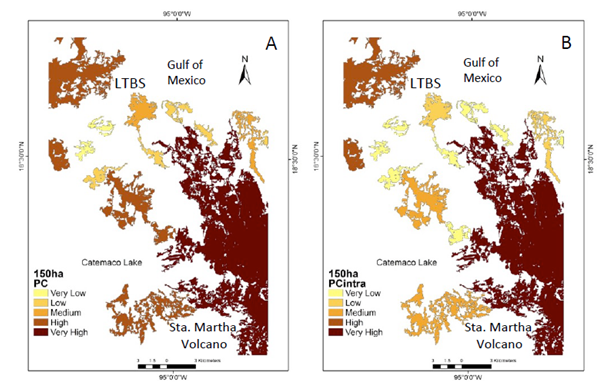
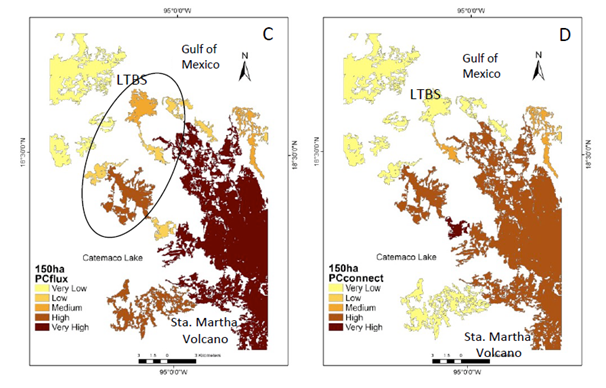
Supplementary Figure 1 Natural vegetation patches > 150 ha (CS1) important forthe maintenance of functional connectivity. (A) Patches are ranked according toPC values. (B) The sub-categories IICintra. (C) IICflux. (D) IICconnect. Circle shows the functional connectivity improvement.
Under the CS1 scenario the IIC showed two main patches with enough available habitat. One patch is located in the south-eastern portion of the study area (Santa Martha Volcano area ca. 15,000 ha), while in the north-western portion there is another area known as the Los Tuxtlas Biological Station (LTBS), as well as the area surrounding the San Martin Tuxtla Volcano, ca. 13,000; (Figure 2a). According to the IIC intra, although these two patches have available habitat of >150 ha (Figure 2b), there is no flux of organisms between them (Figure 2c) because there are no effective connecting elements in the landscape for these two larger areas (Figure 2d). PC indexes, at the >150 ha scenario showed similar results, but PC flux indicated the probability of worthy contribution by central patches (supplementary material Figure 1). Therefore, at this scale most of processes/species remain only in the two large patches. In addition, processes such as seed removal, pollination and plant demography and genetic diversity are severely affected at the landscape level (Table 1).
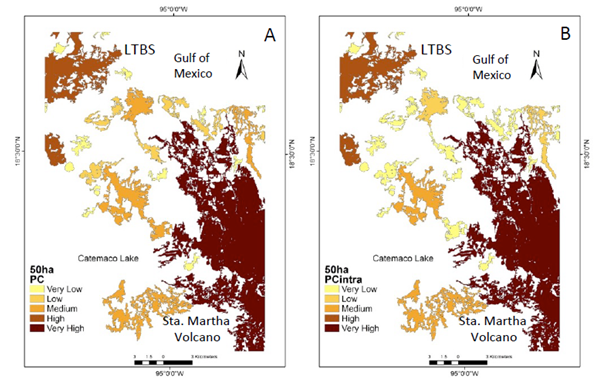
The number of patches (27) in the CS2 scenario show a similar pattern as the >150 ha scenario. However, at this scale the central portion of the study area becomes an important connecting element (Figure 3a−d). When PC indexes were integrated (Figure 4a−b), the probability of flux between patches is higher (supplementary material Figure 2c) mainly due to the newly integrated patches (supplementary material Figure 2a−d). Although while the diversity of insectivorous bird species and some pollinators still persist in the CS2 scenario most other processes have vanished (Table 1).
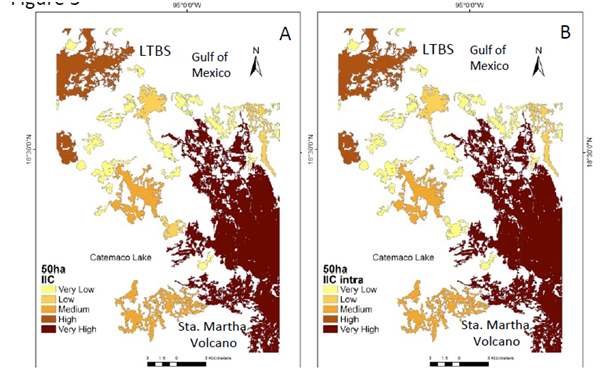
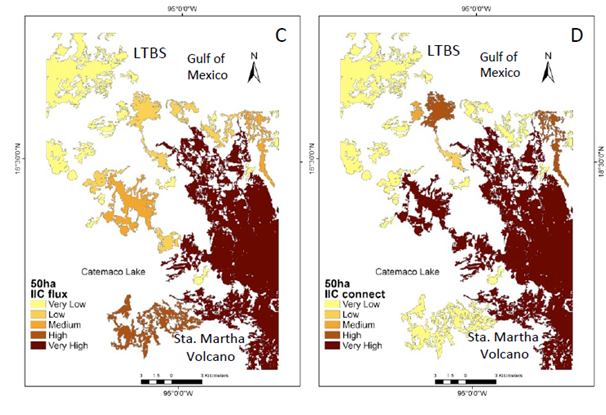
Figure 3 Natural vegetation patches >50 ha (CS2) important for the maintenance of functional connectivity. (A) Patches are ranked according to IIC values. (B) The sub-categories IICintra. (C) IICflux (D) IICconnect.
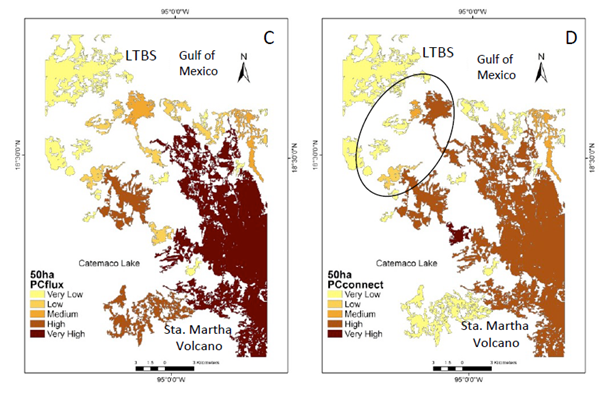
Supplementary Figure 2 Natural vegetation patches >50 ha (CS2) important for the maintenance of functional connectivity. (A) Patches are ranked according to PC values. (B) The sub-categories PCintra. (C) PCflux. (D) PCconnect. Circle shows the functional connectivity improvement.
The CS3 scenario contains 51 patches >25 ha which are the smallest and most isolated in the area under study. Thus, their contribution to general connectivity is poor. Nonetheless, due to their potential contribution in increasing the total area, the patches in the north-western area become connected, as well as the flux and connectivity from the larger patch (Santa Martha volcano) is increased (Figure 4a−d). On the other hand, the PC indexes showed that the probability of use of these patches as suitable habitat is minimal, but the probability for connectivity persists (supplementary material Figure 3a−d). This scenario is the most realistic, however it is highly probable that the majority of the smaller patches have lost their functionality and species pool as indicators showed (Table 1).
The IIC and PC indexes showed that the Los Tuxtlas region is now functionally unconnected. Almost all the processes/species used as indicators showed that have been affected by fragmentation and the loss of functional connectivity. The ranking of species indicated that there are different thresholds in patch sizes to attain at least part of the ecological functionality. For example, processes such as seed removal rates and the loss of wasp and bird diversity were detected in fragments of >25 ha (CS3). The cascade effects because of the alteration of these processes will reverberate up to ecosystem levels.5,33−35 As well, it has been documented that the overgrowth of lianas in patches of this size changes the overall rainforest structure.36,37
The second connectivity scenario (CS2) showed that other ecological processes would be affected such as pollination, tree recruitment and plant demography.38−40 The profound consequences of this disruption have been well documented in the tropics.41,42 Finally, there are those processes/species that due to their size-habitat/functional connectivity ratio their functionality has been almost completely lost (CS1). All of these examples are based on community structure and genetic diversity. Fragmented habitats often have a significant loss of genetic diversity relative to homogeneous habitats. However, this effect is reduced in species with long distance dispersal skills such as birds and bats, but exacerbated in species with low migration rates and a low capacity to cross non-forested matrices.43−45 Under the patch-size/connectivity trade-off, the jaguar, and in general the large-sized terrestrial mammals are some of the best examples. The Los Tuxtlas region has only two patches large enough to support the presence of large felids (jaguar and puma). It is noteworthy that a confirmed footprint of an adult jaguar was collected in a remote area of the Santa Martha volcano, and the local people report incidents of predation on calves by pumas (Puma concolor) in the more remote areas of the San Martin volcano(R. Coates, pers. comm.). However, the functional connectivity is the poorest between these two large patches (cf. CS1).
Patch-size/functional connectivity trade-off
The processes/species analysis suggests a trade-off between patch-size and functional connectivity in fragmented landscapes. Hence, while species and ecological processes need larger patches for their functional maintenance, these patches are more isolated and consequently are less connected. Tropical areas are more frequently under this trade-off,19 thus smaller fragments become more important as conservation and management units.46,47 These small fragments, especially in older fragmented landscapes, could be of high-quality and of great value for biodiversity as they may provide resting places and stepping stones for migratory birds and many seed-dispersing animals.48 Our analysis showed that depending on the scale the value of small fragments escalates, for example in the case of pollination and some seed removal maintenance.
With regard to IIC, our analysis showed that the Los Tuxtlas region has two main natural vegetation patches: one patch represented by the Santa Martha volcano and the second represented by the area of the LTBS and the surrounding forest of the San Martin Tuxtla volcano (only partially included in this analysis). Multiple studies in tropical areas have demonstrated that human activities have led to the reduction of up to 75% of the original rainforest vegetation.18,49 A product of this degradation is the modification in the structure of wildlife communities and ecological processes mediated by them.4 When we partitioned the IIC into the three sub-categories, in the CS1 analysis, the loss of connectivity was evident. For instance, the IICintra detected, in terms of intra-patch connectivity, that only the Santa Martha area is large enough to function as the main patch in the conservation of communities and genetic diversity (within the area analyzed). In addition, the IICconnect showed that this large area was disconnected from the other large area represented by the LTBS, while the IICflux demonstrated that some of the smaller patches analyzed may be very important as stepping-stones to achieve connectivity.
Although the CS2 and CS3 scenarios incorporated many stepping-stones, the fact that fragmentation is paired with habitat loss and the subsequent isolation from continuous forested areas, these factors exert severe pressure on the remaining wildlife.4,50 In addition, the PC index and its subcategories showed the same trends as those of the IIC index. However, the PCflux indicated a higher probability of connection between both of the two main large patches. The PCconnect index, showed the presence of two main paths or corridors through which organisms could reach some other suitable fragments. Although the probabilities may exist, it is important to consider the matrix composition in order to ascertain beforehand, which species are able to cross unsuitable habitats.51 Our indicators analysis allowed us to detect some fragmented areas in the centre of the Los Tuxtlas region, which could be managed to function as biological corridors.
Landscape long-term conservation and management implications
The three connectivity scenarios developed here shed some insight into the functional possibilities for the establishment of landscape management practices. One aspect is the creation of corridors using the present configuration of vegetation patch and at the same time by taking advantage of the capacity of some vertebrate species to cross semi-modified matrices52,53 in order to re-colonize some formerly unconnected patches. One of the main issues is that the majority of the conservation efforts in the tropics are still focused on the extension of areas under official protection rather than implementing protection and management policies for existing remnant patches by integrating the local land owners.25,54 Therefore, taking into account the results of this study, one important long-term conservation aspect should be the implementation of management practices such as agroforestry in pasturelands in order to enhance the potential of the < 50 ha patches for connectivity between the two larger patches where many organisms and ecological processes important in the dynamics of tropical rainforest are still present.
Recommendations for conservation
The conservation status of tropical forests seems to be more threatened each day and larger areas of secondary vegetation that have already lost their original structural features, dominate tropical landscapes.3,55,56 Therefore, we suggest three main actions that could be applied in other tropical areas under the same situation: 1) the implementation of relatively simple wildlife monitoring systems, 2) the integration of the local people in productive and sustainable projects such as agroforestry and animal husbandry and 3) the promotion of collaboration between local landowners and scientists to achieve common goals which will generate pertinent information on ecological processes in which many organisms are involved in order to arrest the current and future extinctions caused by fragmentation and therefore will contribute significantly to the long-term conservation and management of the remaining tropical rainforests.
Ecological processes are directly affected by loss of functional connectivity. However, depending on the patch size the effects could be clearly present or with manageable effects, therefore we proposed that even smaller fragments could be managed in order to get ecological functional corridors, which improve the chances of maintain processes that support the long-term tropical rain forest diversity.
Authors wish to thank the Instituto de Biología, UNAM, for providing the funding for this study. We also thank the entire staff of the Estación de Biología Tropical Los Tuxtlas. AR-M and GG-G were granted with a postdoctoral fellowship by CONACyT.
Author declares there is no conflict of interest in publishing the article.

©2018 Gutiérrez-Granados, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.