eISSN: 2575-906X


Research Article Volume 1 Issue 5
1Postgraduate Program in Entomology, National Institute of Amazonian Research, Brazil
2Biodiversity Coordination, National Institute of Amazonian Research, Brazil
Correspondence: Bruno Garcia de Oliveira, National Institute of Research of the Amazon, Av. Bem Te VI, 8-406 - Petr
Received: September 28, 2017 | Published: December 6, 2017
Citation: Oliveira GB, Gadelha SS, Teles BR. Composition of braconidae (hymenoptera) fauna in citrus orchards surrounded by Amazonian secondary forest. Biodiversity Int J. 2017;1(5):34-39. DOI: 10.15406/bij.2017.01.00025
The Braconidae composition of two Citrus orchards surrounded by Amazon rainforest was investigated. To compare the composition of Braconidae in orchards and forest were installed eighteen modified Malaise traps distributed in a gradient that started inside the orchard 80 meters from the edge extending up to 80meters from the border inside the forest. The composition of Braconidae differed between forest and orchard, abundance in orchard was lower when compared with the forest and the diversity were higher inside forest. Differences found in the composition is associated with factors such as the presence of hosts in the environment. Was found some potential genera like Utetes, and Pholetesor that can be used in biological control of citrus pests.
Keywords: Parasitoid, ichneumonoidea, citrus, amazon, biological control
Brazil is the world’s largest producer of oranges [Citrus sinensis L. Osbeck (Sapindales, Rutaceae)] in the world, making the Citrus industry one of the most important crop for Brazilian agro economic. In the Amazon region the Citrus industry is growing, with crops concentrated mainly in Manaus, Iranduba, Rio Preto da Eva and Manacapuru the varieties “Pera-Lima” and “Valencia” are planted in about 2.700 ha1 and an example of this growth is the production doubled when compared with five years ago.2
Braconidae is the second largest family of Hymenoptera,3 with about 46 subfamilies and nearly 20,000 valid species in the world.4 They are mostly parasitoids of insects, especially larvae of Lepidoptera, Coleoptera and Diptera.5 As their hosts are invariably killed during larval development process, these parasitoids play an important ecological role in regulating populations of other insects many of which could be damaging to agrosystems if their numbers were not contained and that is being applied increasingly in programs of biological control.6 In South and Southeast region fruit flies are major pest of citrus7‒9 and they can be controlled by some species of Braconidae such as Doryctobracon areolatus (Szépligeti) and Diachasmimorpha longicaudata (Ashmead). Another example of the importance of Braconidae for citrus is some aphids that are controlled by Lysiphlebus testaceipes (Cresson) but very little is known about the diversity of Braconidae in the Amazon region.Several works on diversity of fruit flies and their parasitoids contributed not in a direct way to the knowledge of Braconidae in the Amazon region10‒17 Those works lack on information about Braconidae that is more specific about identification and ecology. There is only three works treating Braconidae diversity in more specific way in Amazonian region and they are not associated with crops. Feitosa et al.18, Querino et al.19 studied the diversity of parasitoids in general and Gadelha et al.20 studied the diversity of Braconidae in a secondary forest.Regarding the growth of citrus industry on Amazonas state and the importance of Braconidae for the biological control of citrus pests this work aimed to compare the composition of Braconidae fauna on two Citrus orchards and in the surrounding secondary forests and provide information about Braconidae taxonomic fauna as a base for future studies on the implementation of biological control in Amazonian Citrus plantations.
The study was carried out in two Citrus orchards surrounded by secondary forest located near Rio Preto da Eva City in the Amazonas State. The orchards were located between kilometers 80 and 87 of the AM-010 freeway. Both orchards measured approximately 15 hectares planted with C. sinensis, variety "Pera", and were surrounded by secondary Amazon forests with similar floristic composition. In the seven years old Citrus Orchard Aprisco Pasárgada (SAP, 2°42'24.65"S, 59°42'52.56" W), trees were planted with space of 6x7m from each other, corresponding to a total density of 238 plants per hectare. In six years old Citrus orchard Santa Terezinha (SST, 2°40'16.68"S, 59°39'24.24"W), trees were planted with space of 5x8meters from each other, corresponding to a total density of 250 plants per hectare.
The adult parasitoids were collected using a modified Malaise Trap21 (Figure 1). The modifications of the trap consisted in the top being a white pyramid with a base of 1m² square made of a very fine mesh and a black crossed septum of 2m below touching the ground. On top of the trap a glass collector was attached, filled with 700ml of a solution composed of 70% alcohol and glycerin in the ratio of 9 to 1, respectively. The collections of Braconid were made during nine months, with the glass collector removed each fifteen days giving thus two collections per month from September 2013 to May 2014. Nine traps were used in each orchard starting at 80 m from edge inside the orchard until 80 meters from the edge inside the forest. Traps were not installed in straight line to keep at least 60 m away from each trap (Figure 2). The specimens were identified in genus level based on identification keys proposed by Wharton et al.22, Marsh23 using a stereomicroscope. The parasitoids analyzed were deposited at the Invertebrate Collection of the National Institute of Amazonian Research (INPA).
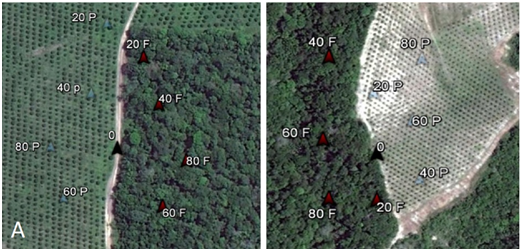
Figure 2 Disposition of Malaise traps inside A) Citrus orchard ApriscoPasárgada B)Citrus orchard Santa Terezinha.The numbers in front of the letters represents the distance in meters from the edge and the letters (P) are the traps inside orchard and (F) inside forest.
For abundance data, the sums of all traps in each orchard and the sum of all traps in each secondary forest were used. The analysis of variance was done by adding all trapsper vegetation type (orchard or forest). The composition analysis was made using Principal Coordinates Analysis (PCoA) considering data of each trap inside each vegetation type. A posteriori test of analysis of variance was performed using the two first axes of the ordination provided by PCoA. Levels of significance were considered below 0.05.All analyses and graphics were run in the R version 2.1.1 environment for statistical computing,24 with Vegan and MASS packages.
A total of 586 individuals were collected, distributed in 21 subfamilies and 65 genera. The four most abundant subfamilies were Doryctinae(176) Microgastrinae(109) Hormiinae(79) and Rogadinae(62) (Table 1). Abundance inside the forest, in the edge of forest and inside orchard were 315,77 and194 respectively. The edge abundance was different from forest and orchard and forest abundance was different from orchard (Figure 3). The most abundant families in forest were Doryctinae, Horminiae and Rogadinae and in orchard were Microgastrinae and Braconinae. The PCoA showed difference in the composition of forest and edge composition. Forest composition was similar to the edge (Figure 4). Moreover, exclusivity of some subfamilies in each vegetation type were found (Figure 5).
Subfamily/Genus |
Aprisco Pasárgada |
Santa Terezinha |
|||||||||||||||||
80P |
60P |
40P |
20P |
Borda |
20M |
40M |
60M |
80M |
80P |
60P |
40P |
20P |
Borda |
20M |
40M |
60M |
80M |
||
Agathidinae |
Alabagrus |
X |
|||||||||||||||||
Bassus |
X |
X |
X |
||||||||||||||||
Alysiinae |
Aphaereta |
X |
X |
X |
X |
X |
|||||||||||||
Asobara |
X |
||||||||||||||||||
Dinotrema |
X |
X |
X |
X |
X |
X |
X |
X |
|||||||||||
Phaenocarpa |
X |
X |
|||||||||||||||||
Betylobraconinae |
Jannya* |
X |
X |
||||||||||||||||
Blacinae |
Dyscoletes* |
X |
|||||||||||||||||
Braconinae |
|||||||||||||||||||
Braconinae |
Bracon |
X |
X |
X |
X |
X |
X |
X |
X |
||||||||||
Hemibracon |
X |
X |
|||||||||||||||||
Brachistinae |
Eubazus |
X |
|||||||||||||||||
Cenocoelinae |
Capitonius |
X |
|||||||||||||||||
Cheloninae |
Chelonus |
X |
X |
X |
X |
X |
|||||||||||||
Phanerotoma |
X |
X |
|||||||||||||||||
Pseudophanerotoma |
X |
X |
X |
||||||||||||||||
Doryctinae |
Barbalhoa |
X |
|||||||||||||||||
Ecphylus |
X |
X |
X |
X |
|||||||||||||||
New g. E |
X |
||||||||||||||||||
Heterospathius |
X |
X |
X |
X |
X |
X |
|||||||||||||
Heterospilus |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
|||||
Johnsonius |
X |
||||||||||||||||||
Leluthia |
X |
||||||||||||||||||
Leptodoryctes |
X |
X |
X |
||||||||||||||||
Neoheterospilus |
X |
X |
X |
X |
X |
X |
|||||||||||||
Notiospathius |
X |
X |
X |
X |
X |
X |
X |
||||||||||||
Pioscelus |
X |
||||||||||||||||||
Stenocorse |
X |
||||||||||||||||||
Euphorinae |
Aridelus |
X |
|||||||||||||||||
Centistes |
X |
||||||||||||||||||
Euphoriella |
X |
X |
|||||||||||||||||
Plynops |
X |
X |
|||||||||||||||||
Exothecinae |
Colastes |
X |
|||||||||||||||||
Gnamptodontinae |
Gnamptodon |
X |
|||||||||||||||||
Pseudognaptodon |
X |
||||||||||||||||||
Homolobinae |
Exasticolus |
X |
X |
X |
X |
X |
|||||||||||||
Hormiinae |
Allobracon |
X |
X |
X |
X |
X |
|||||||||||||
Hormius |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
||||||||
Ichneutinae |
Lispixys |
X |
|||||||||||||||||
Macrocentrinae |
Dolichozele |
X |
|||||||||||||||||
Mendesellinae |
Epsilogaster |
X |
|||||||||||||||||
Microgastrinae |
Alphomelon |
X |
X |
X |
|||||||||||||||
Apanteles |
X |
X |
X |
X |
X |
X |
X |
X |
|||||||||||
Cotesia |
X |
X |
X |
X |
|||||||||||||||
Distatrix |
X |
||||||||||||||||||
Glyptapanteles |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
||||||||
Hypomicrogaster |
X |
X |
|||||||||||||||||
unidentified males |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
||||
Papanteles |
X |
||||||||||||||||||
Parapanteles |
X |
X |
X |
||||||||||||||||
Pholetesor |
X |
X |
X |
||||||||||||||||
Pseudapanteles |
X |
X |
|||||||||||||||||
Wilkinsonellus* |
X |
||||||||||||||||||
Xanthomicrogaster |
X |
X |
|||||||||||||||||
Miracinae |
Mirax |
X |
X |
||||||||||||||||
Opiinae |
Utetes |
X |
X |
X |
X |
X |
X |
||||||||||||
Orgilinae |
Bentonia |
X |
X |
X |
|||||||||||||||
Stantonia |
X |
||||||||||||||||||
Pambolinae |
Pambolus |
X |
X |
X |
X |
X |
X |
||||||||||||
Rhyssalinae |
Acrisis* |
X |
|||||||||||||||||
Oncophanes* |
X |
||||||||||||||||||
Rogadinae |
Aleiodes |
X |
X |
X |
X |
||||||||||||||
Choreborogas |
X |
X |
X |
X |
X |
||||||||||||||
Cystomastax |
X |
X |
|||||||||||||||||
Rogas |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
|||||||||
Stiropius |
X |
X |
X |
||||||||||||||||
Yelicones |
X |
X |
|||||||||||||||||
Table 1 Presence of the genus within the orchards.
*New records for Brazil
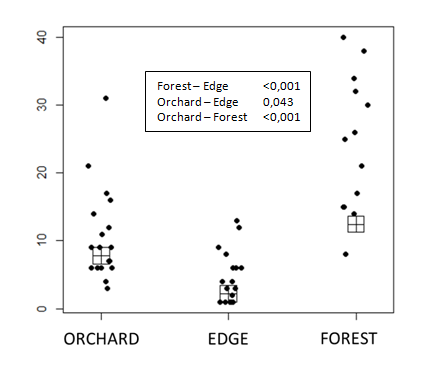
Figure 3 Abundance of Braconidae in orchards surrounded by secondary forest. * means p value below 0.05.
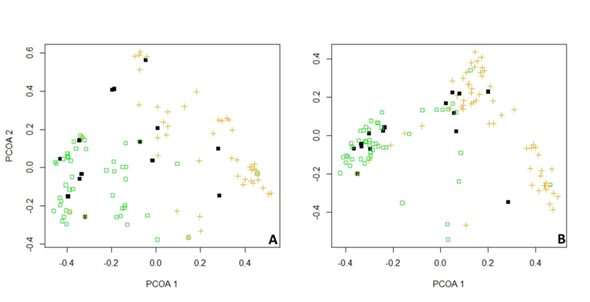
Figure 4 Principal coordinates analysis (PCoA) with data from both areas. A) Subfamily level. B) Genus level. (orange cross =orchard; black square =edge; green square =forest).
Insect composition is influenced by several factors such as weather conditions, presence of hosts (in case of parasitoids), and presence of food and also by types of vegetation. Therefore, a monoculture plantation, as it happens in the Citrus orchards, can favor the presence of a limited group of phytophagous insectsin which consequently favors the presence of a specific niche of parasitoids.25‒27 This was the case of groups like Microgastrinae, Orgilinae, Braconinae, Homolobinae and Agathidinae, which occurred mainly in the orchard. These groups are generally associated with parasitism of larvae of Lepidoptera.22,28 Even though wasn’t made any quantitative survey of Lepidoptera larvae we were able to observe these individuals in the orchard during the sampling period. This may have contributed to the presence of the subfamilies cited. A more specific example is the genus Pholetesor Mason, 1981 (Microgastrinae) whose most specimens were collected in the orchard. This genus has been recorded by Santos et al.29 as parasitoid of Phyllocnistis Zeller, 1848, which is one of the main pests of citrus, and the species Phyllocnistis citrella Stainton, 1856, was present in the orchard.
Tropical rain forests have a greater diversity of plants and insects with a more bearable environment if compared to a Citrus orchard. This environment can create exclusivity of some groups that present some specificity of niches and habits of life, as Doryctinae that are mainly parasitoid of wood-boring beetles23 and attack larvae of beetles inside stems. The higher diversity of Doryctinae inside forest is explained by the high diversity of trees found inside forest in contrast with the low diversity inside orchard (only Citrus trees). Hormiinae and Pambolinae, as another example, were present almost exclusive in the forest. These subfamilies have the habit of parasitize insects that can hide some way inside plant tissue (leaf miners, gall or borers).30 Hormius Nees, 1819 (Hormiinae), for example, is parasitoid of Gelechiidae, Tortricidae and Coleophoridae all Lepidoptera,30 which should certainly occur inside forest but not inside orchard, where the only leaf miner found was Phyllocnistis (Gracillariidae). Pambolus Haliday, 1836 (Pambolinae) is parasitoid of Coleoptera30 which has been little observed in the orchard areas.
There are only few studies with ecological observations regarding the Braconidae fauna in the Amazonian region. In this study, the number of Braconidae specimens (586) were less than the approximately nine thousand collected by Feitosa et al.18 with three malaise traps (type: Gressit and Gressit, 1962) for a year, and less than almost three thousand collected by Querino et al.19 using twenty-four suspended traps for a year. Our numbers surpassed only Gadelha et al.20 in which were collected 377 individuals using six malaise traps (type: Towns 1972) for a year. In addition, using 18 modified malaise traps for eight months we were able to record a higher number of subfamilies (21) and genera (65) than that showed by Gadelha et al.20 Furthermore, six subfamilies (Betylobraconinae, Blacinae, Euphorinae, Icneutinae, Mendesellinae and Miracinae) and 38 genera weren’t recorded in any of the studies cited above. Were found some potential groups for biological control of citrus pests, mainly inside orchard, for instance, Utetes Forester (Opiinae). This genus uses fruit-infesting tephritids as its main host31 and despite this type of pest still not attack Citrus orchards in the northern region of Brazilits presence in the orchard is an indicative of a possible natural defense against tephritids, if it ever occurs.
The difference of Braconidae composition was caused by the presence of groups of agricultural importance on orchard and it can be attributed by the difference of vegetation between orchard and forest. Was collected some groups that are promising for biological control and if better studied it is possible to develop a regional biological control to reduce the use of pesticides. The distribution of some genera was expanded to Brazil, and those new records occurred mainly inside forest, showing the importance of maintain the forests and it should be taken in account a manner to keep most of the forest around orchard due to the diversity of genera that have been found.
The authors would like to thank the owners of the orchards studied, CAPES for the scholarship provided for the students, INPA for the infrastructure, EMBRAPA for the financial support in the collections and Dr. Marcos Vinícius Bastos Garcia.
Authors declare there is no conflict of interest in publishing the article.

©2017 Oliveira, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.