eISSN: 2575-906X


Research Article Volume 1 Issue 4
Department of Environmental Sciences, Hemvati Nandan Bahughuna Garhwal University, India
Correspondence: Ramesh C Sharma, Department of Environmental Sciences, Hemvati Nandan Bahughuna Garhwal University, Srinagar, Garhwal, 246174, Uttarakhand, India
Received: September 29, 2017 | Published: November 17, 2017
Citation: Sharma RC, Singh S. Macrophytes of sacred Himalayan lake Dodi Tal, India: quantitative and diversity analysis. Biodiversity Int J. 2017;1(4):137-144. DOI: 10.15406/bij.2017.01.00020
The present study was conducted for quantitative and diversity analyses of aquatic macrophytes dwelling littoral zone of sacred lake Dodi Tal (3,075 masl) situated in Garhwal Himalaya in summer and winter seasons of 2015 and spring of 2016. A total of 45 macrophyte species belonging to 29 families and 34 genera were reported. These macrophytes varied in different morphological groups- emergents; submerged; rooted-floating leaf type; and free floating. Maximum number of species were represented by emergents (30) followed by submerged (10), rooted- floating leaf type (3) and free floating (3) macrophytes. Two emergent species (Renunculus letus, Myriophyllum verticillatum) of macrophytes dominated during all the three seasons. Renunculus letus was dominant in summer (IVI=10.40) and spring (IVI=12.61) seasons, while Myriophyllum verticillatum was dominant in winter season (IVI=12.94). The highest species diversity (H’) of macrophytes was observed during summer (3.77) followed by spring (3.53) and winter (3.40) season. The abundant growth of aquatic macrophytes in the littoral zone of the lake may be due to various anthropogenic activities of the tourists in the riparian zone of the Dodi Tal. The huge growth of macrophytes during springs and summer seasons may be correlated with huge influx of tourists during this period.
Keywords: Garhwal himalaya, high altitude lake, importance value index (ivi), macrophytes, species diversity
Biodiversity is one of the most important characteristics of the aquatic ecosystem for maintaining its stability and resilience.1 Aquatic macrophytes are macroscopic forms of aquatic vegetation, including macroalgae, mosses, ferns and angiosperms found in aquatic habitat. They have evolved from many diverse groups and often demonstrate extreme flexibility in structure and morphology in relation to changing environmental conditions.2 Macrophytes of freshwater ecosystems have diverse roles to play in the structure and functioning of these ecosystems.3 Macrophytes are considered as an important component of the aquatic ecosystem not only as the habitat and food source for aquatic life, but also act as an efficient accumulator of heavy metals4‒7 and as an important participant in the natural processes of water self-purification of water.8 Aquatic macrophytes represent the most important biotic component of the littoral zone of the lake ecosystem.9 Different forms of macrophytes exhibit seasonally variable growth patterns.3 Number of species and importance values of macrophytes, determines the species diversity of a community.10 Importance Value Index (IVI), a quantitative parameter, is useful, for providing the details on density and frequency of a species in relation to community.11 However, the species diversity is a useful index for the comparison of communities under the influence of anthropogenic disturbances.12
A considerable contribution on the aquatic macrophytes of India has been made.13‒19 Although, some scattered reports are available on the lakes of Garhwal Himalaya.20,21 No sincere attempt has been made on the diversity and quantitative analyses of the macrophytes of the sacred Himalayan lake of Dodi Tal. The present study will be important for the conservation and management of the sacred lake through regulating the influx of the tourists to this Himalayan lake. As the quantity and diversity of macrophytes are the important parameters for assessing the health of the ecosystem.
Study area
The Garhwal Himalaya is dotted with many high altitude lakes. The Dodi Tal is a very important high altitude sacred lake of the Garhwal Himalaya. The lake is dedicated to lord Ganesha (Dundi Maharaj), the most respected God of Indian mythology. The lake is known as the birth place of lord Ganesh, the younger son of lord Shiva. Therefore, it is worshiped by a large number of devotees. It is located between latitude 30°52’31.99 N and longitude 78°31’12.47” E at an altitude of 3,075m a.s.l. in Uttarkashi district of Uttarakhand state, India. It is surrounded by dense oak (Quercus) forest and mountains at three sides. The lake is fed by two snow fed streams originated from an alpine meadow - Bakariya Khagi Pass (Figure 1). The only outlet of Dodi Tal partially forms the river Asi Ganga, an important tributary of Bhagirathi river. Dodi Tal is semi triangular in shape and measures 248.22m long and 151.99m wide at its broadest points. The littoral zone of this high altitude lake is very prominent. However, the littoral zone of this lake is very shallow at the water level fluctuates seasonally. Dodi Tal has a maximum depth of 19.97m with an average depth of 9.98m.
The surrounding floristic environment of Dodi Tal is mainly represented by temperate vegetation. The upper zone is dominated by some common tree species like Pinus spp L., Quercus spp L., Rhododendron spp L., Abies pindrow Royle , Lyonia ovalifolia (Wallich ) Drude, Alnus nepalensis D. Don, Cedrus deodara (Roxb. ex D. Don) , Picea smithiana (wallich) Boissier and Taxus spp L., Acer spp L., Juglans regia L. , and Betula utilis D.Don. The beneath narrative is dominated by the shrubs like Berberis aristata DC., Salix elegans Wallich ex Anderson, Aconitum spp. L., Picrorhiza kurrooa Royle ex Benth , Nardostachys grandiflora DC and Dactylorhiza hatagirea D.Don along with some climbers like Clematis grata Wallich and Clematis gouriana Roxb. ex DC. The area also supports a good faunal diversity such as Himalayan Musk deer (Moschus chrysogaster Hodgson,), Himalayan tahr (Hemitragus jemlahicus C.H. Smith), Snow leopard (Panthera uncia Schreber,), Himalayan goral (Naemorhedus goral Hardwicke), Asiatic Black bear (Ursus thibetanus G. [Baron] Cuvier), Common leopard (Panthera pardus Linnaeus), Hanuman langur (Semnopithecus entellus Dufresne,) and Rhesus macaque (Macaca mulatta Zimmermann).
Macrophyte sampling
Quantitative and diversity analyses of macrophytes of Dodi Tal was carried out in the littoral zone during summer (August 2015), winter (December 2015) and spring (May 2016) seasons. A random sampling along several transects with the help of a 1m2 light wooden quadrat was undertaken. The quadrat size was determined by the species area curve method.22 The length of transect and numbers of quadrats in each transect within each sampling unit were adjusted according to the depth of the littoral zone. The centre of the Dodi Tal was not accessible for quadrat study due to its high depth. All the macrophytes were counted by hand picking. A total of 60 quadrats were studied during each season - 15 quadrats from each sampling sites. The plant species were identified with the help of standard literature23‒31 and visual inspection by plant taxonomists.
Importance Value Index (IVI) was calculated by totaling the relative values of density, frequency and cover (by visual estimation). Diversity index (H’) was calculated following the Shannon & Weiner32 formula: H’=- Σpi* log pi; where, pi = the proportion of importance value of the ith species, pi = ni/N, ni is the importance value of i-th species and N is the importance value of all the species.22 The Pielou’s evenness index is also an important component of the diversity indices. Pielou’s evenness J=H′ ln (S) is a measure of equitability, a measure of how evenly the individuals are distributed among the different species. Thus Pielou’s Evenness33 commonly expressed by J’ was also calculated.
A total of 45 species of macrophytes belonging to 34 genera and 29 families were recorded from the littoral zone of the high altitude lake, Dodi Tal of Garhwal Himalaya (Table 1). These aquatic macrophytes are used by local people as food, fodder and medicinal purposes. Maximum number of species belonged to emergent (30 species) followed by submerged (10 species), rooted-floating leaf type (3 species) and free floating (3 species) macrophytes. The Cyperaceae family was found to be highly dominant in the emergent macrophytes. While no single family showed dominance in the free floating ones; whereas, the family Potamogetonaceae was found to be highly dominant in both submerged as well as rooted floating leaf types. Macrophytes dwelling the littoral zone of Dodi Tal and their common name, category of uses, have been presented in (Table 1). The composition of macrophytes in the littoral zone of the Dodi Tal is clearly visible in (Figure 2) (Figure 3).
Category of Macrophytes |
Species |
Family |
Common Name |
Uses |
Emergents |
Bidens pilosaL. |
Asteraceae |
Black jack, spanish needle |
Food or medicine |
Bidens cernuaL. |
Compositae |
Nodding bur - marigold (sunflower) |
Medicinal herb, Folk medicine, |
|
Carex wallichiana Spreng. |
Cyperaceae |
Sedges - |
Food, livestock hay |
|
Caladium mariscusL. |
Cyperaceae |
Swamp sawgrass |
Livestock hay |
|
Dryopteris wallichiana (Spreng.) Hyl. |
Dryopteridaceae |
Alpine wood fern |
Medicinal anthelmintic |
|
Dryopteris odontoloma(Moore) C. Chr. |
Dryopteridaceae |
Wood fern |
Medicinal anthelmintic |
|
Eleocharis uniglumis (Link) Schult. |
Cyperaceae |
Slender spike rush |
Food, livestock hay |
|
Eleocharis palustris(L.) Roem. & Schult. |
Cyperaceae |
Common spike rush |
Food, livestock hay |
|
Galium albumMill. |
Rubiaceae |
- |
- |
|
Hippuris vulgarisL. |
Hippuridaceae |
Common mare’s tail |
Herbal medicine , curing stomach ulcers, soothing inflammation skin |
|
Lycopus europas L. |
Lamiaceae |
Bugleweed, Gypsywort |
Medicinal , astringent, cosmetic, douche, narcotic, refrigerant |
|
Menyanthes trifoliateL. |
Menyanthaceae |
Bogbean, buckbean |
Bitter and strong taste, schnapps |
|
Myriophyllum verticillatumL. |
Haloragaceae |
Whorled water- milfoil |
Good water oxygenator |
|
Nasturtium offcinale L. |
Brassicaceae |
watercress |
Edible, rich in vitamin k,vitamin c, riboflavin, calcium, vitamin B6 |
|
Phargmites australis(Cav.) Trin. ex Steud. |
Poaceae |
Common reed beds, perennial grass |
Great wood structure, edible |
|
Polygonum hydropiper (L.) Spach |
Polygonaceae |
Water pepper |
Active ingredients, essential oil, edible |
|
Ranunculus letusL. |
Ranunculaceae |
Leaved buttercup |
|
|
Ranunculus secleratusL. |
Ranunculaceae |
Celery - leaved buttercup |
Blisters on human skin |
|
Sagittaria sagittifoliaL. |
Alismataceae |
Arrowhead |
Tuber is edible, medicinal |
|
Scirpus lacustris(L.) Palla |
Cyperaceae |
Lakeshore bulrush, common club rush |
- |
|
Scirpus triqueter(L.) Palla |
Cyperaceae |
Deergrass, grassweed |
- |
|
Scirpus palustris(L.) Roem. & Schult. |
Cyperaceae |
Common spike - rush |
- |
|
Scirpus maritimusL. |
Cyperaceae |
Club- rush |
- |
|
Sium latijugumC. B. Cl. |
Apiaceae |
Large leaflet water parsnip |
- |
|
Sparganium erectum L. |
Typhaceae |
Branched bur reed |
- |
|
Sparganium ramosumHuds. |
Sparganiceae |
Bur reed |
|
|
Rumex maritimusSm. |
Polygonaceae |
Golden dock |
|
|
Typha angustatifoliaL. |
Typhaceae |
Reedmace, bulrush |
Edible like flour |
|
Veronica anagallisL. |
Scrophulariaceae |
Blue water speedwell |
- |
|
Rooted floating - leaf type |
Marsilea quadrifoliaL. |
Marsileaceae |
Four leaf clover |
Edible, antiflammntory, treat snakebite and applied abscesses |
Potamogeton natans L. |
Potamogetonaceae |
Broad – leaved pondweed |
- |
|
Potamogeton nodosusPoir |
Potamogetonaceae |
Longleaf pondweed |
- |
|
Submerged |
Chara delicatulaC. Agardh |
Characeae |
Green algae |
Recreational use |
Ceratophyllum demersumL. |
Ceratophyllaceae |
Hornwort, cootail |
Popular aquarium plant, kill snails |
|
Hydrilla verticillata(L.f.) Royle |
Hydrocharitaceae |
Hydrilla, waterthyme |
Phytoremediation, Recreational use |
|
Myriophyllum spicatumL. |
Haloragaceae |
Spiked water milfoil |
- |
|
Nitellaspp. |
Characeae |
Green algae |
- |
|
Najasspp. |
Hydrocharitaceae |
Waternymphs, naiads |
|
|
Potamogeton crispusL. |
Potamogetonaceae |
Curled pondweed or curly leaf pondweed |
- |
|
Potamogeton lucensL. |
Potamogetonaceae |
Shining pondweed |
- |
|
Potamogeton pectinatusL. |
Potamogetonaceae |
|
- |
|
Ranunculus trichophyllusChaix |
Ranunculaceae |
Threadleaf crowfoot |
- |
|
Free - floating |
Riccia fluitansL. |
Ricciaceae |
Floating crystalwort |
- |
Lemnaspp. |
Lemnoideae |
duckweed |
Biopharmaceuticals |
|
Salvinia natans(L.) All |
Salviniaceae |
Floating fern , floating moss |
- |
Table 1 Aquatic macrophytes (category, common name, family and uses) dwelling littoral zone of sacred Himalayan lake Dodi Tal.

Figure 3 Macrophytes Composition of Dodi Tal, 1. Emergent species A: Ranunculus letus;B: Myriophyllum verticillatum;C: Bidens Pilosa;D: Bidens cirnua;E: Dryopteris wallichiana;2. Rooted - floating leaf type species F: Marsilea quadrifolia;G: Potamogeton natans;H: Potamogeton nadosus; 3. Submerged species I: Hydrilla verticillata;J: Chara spp. 4. Free floatingK: Riccia fluitans;L: Lemna species.
Quantitative analysis of freshwater macrophytes
The emergent macrophytes grow intensively in the littoral zone up to depth of 0.10-0.30m except in the zones of flowing water (close to the outlet). The dominant ones among the emergent group were Myriophyllum verticillatum, Ranunculus letus, Sium latijugam, Sparganium erectum, Galium hartifolium and Sagittaria sagittifolia. The second layer is formed by the rooted floating – leaf type upto a depth of 0.30-0.50 m with predominance of Potamogeton nodosus, Potamogeton natans, Marsilea quadrifolia and Potamogeton crispus. The dominant submerged macrophytes growing abundantly in the limnetic zone include Chara spp., Hydrilla verticillata, Najas spp. Nitella spp. and Potamogeton crispus. The number of aquatic macrophyte species were higher during summer (45) and spring (35) seasons and low during winter (31) season (Table 2). The percentage composition of different forms of macrophytes is presented in (Figure 4). Emergent species contributed (65%) to the total macrophytes, followed by submerged (22%), free floating (7%) and Rooted - floating leave type (6%) (Figure 4).
Species, Categorized by Growth Form |
Importance Value Index (IVI) in |
|||
Emergent |
Summer |
Winter |
Spring |
Average |
Bidens pilosa |
9.50 |
8.90 |
7.22 |
8.54 |
Bidens cernua |
9.60 |
0.00 |
9.44 |
6.35 |
Carex wallichiana |
7.40 |
0.00 |
6.46 |
4.62 |
Caladium mariscus |
8.60 |
8.70 |
5.11 |
7.47 |
Dryopteris wallichiana |
6.70 |
9.66 |
9.61 |
8.66 |
Dryopteris odontoloma |
7.40 |
9.79 |
8.45 |
8.55 |
Eleocharis uniglumis |
7.20 |
9.24 |
0.00 |
5.48 |
Eleocharis palustris |
5.90 |
9.64 |
0.00 |
5.18 |
Galium hertifolium |
6.10 |
0.00 |
11.47 |
5.86 |
Hippuris vulgaris |
6.20 |
11.05 |
8.45 |
8.57 |
Lycopus europas |
5.60 |
8.42 |
7.27 |
7.10 |
Menyanthes trifoliate |
5.80 |
0.00 |
8.15 |
4.65 |
Myriophyllum verticillatum |
7.30 |
12.94 |
6.98 |
9.07 |
Nasturtium offcinale |
6.50 |
0.00 |
8.24 |
4.91 |
Persicaria amphibia |
5.90 |
10.36 |
0.00 |
5.42 |
Phargmites australis |
5.10 |
0.00 |
0.00 |
1.7 |
Polygonum hydropiper |
5.40 |
0.00 |
0.00 |
1.8 |
Ranunculus letus |
10.40 |
0.00 |
12.61 |
7.67 |
Ranunculus secleratus |
5.60 |
0.00 |
7.76 |
4.45 |
Sagittaria sagittifolia |
7.20 |
0.00 |
11.33 |
6.18 |
Scirpus lacustris |
7.80 |
4.00 |
0.00 |
3.93 |
Scirpus triqueter |
3.80 |
0.00 |
0.00 |
1.27 |
Scirpus palustris |
8.50 |
9.99 |
0.00 |
6.16 |
Scirpus maritimus |
4.50 |
8.92 |
0.00 |
4.47 |
Sium latijugum |
5.90 |
11.70 |
9.16 |
8.92 |
Sparganium erectum |
5.90 |
11.52 |
7.59 |
8.34 |
Sparganium ramosum |
4.70 |
6.47 |
0.00 |
3.72 |
Rumex maritimus |
7.50 |
10.67 |
9.16 |
9.11 |
Typha angustatifolia |
4.70 |
5.01 |
0.00 |
3.24 |
Veronica anagallis |
3.50 |
0.00 |
6.88 |
3.46 |
Total |
196.20 |
166.98 |
161.34 |
174.84 |
Rooted Floating – Leaf Type |
|
|
|
|
Marsilea quadrifolia |
8.10 |
0.00 |
9.63 |
5.91 |
Potamogeton natans |
6.00 |
11.70 |
9.16 |
8.95 |
Potamogeton nodosus |
6.80 |
12.04 |
8.03 |
8.96 |
Total |
20.90 |
23.74 |
26.82 |
23.82 |
Submerged |
|
|
|
|
Chara delicatula |
9.70 |
12.70 |
11.02 |
11.14 |
Ceratophyllum demersum |
7.80 |
6.58 |
7.87 |
7.42 |
Hydrilla verticillata |
8.20 |
12.04 |
10.40 |
10.2 |
Myriophyllum spicatum |
6.90 |
9.76 |
7.51 |
8.06 |
Nitella spp. |
7.40 |
10.58 |
9.63 |
9.20 |
Najas spp. |
4.50 |
10.70 |
7.22 |
7.47 |
Potamogeton crispus |
7.60 |
10.23 |
8.89 |
8.91 |
Potamogeton lucens |
7.70 |
9.66 |
7.37 |
8.24 |
Potamogeton pectinatus |
0.00 |
9.76 |
6.39 |
5.38 |
Ranunculus trichophyllus |
5.80 |
0.00 |
11.11 |
5.64 |
Total |
65.60 |
92.01 |
87.41 |
81.67 |
Free - Floating |
|
|
|
|
Riccia fluitans |
5.00 |
6.31 |
8.46 |
6.59 |
Lemna spp. |
7.20 |
10.93 |
6.78 |
8.30 |
Salvinia natans |
5.50 |
0.00 |
9.26 |
4.92 |
Total |
17.70 |
17.24 |
24.50 |
19.81 |
Grand total |
300.4 |
299.97 |
300.0 |
300.1 |
Table 2 Seasonal variations in important value index (IVI) of different growth forms of macrophytes of Dodi Tal.
Importance value index (IVI) of freshwater macrophytes
The dominance of species by growth forms on the basis of IVI value is presented in (Table 2). Emergents were the most dominant form throughout the year. The emergent macrophytes grow intensively in the littoral zone except near the outlet region. Seasonally, IVI of emergent macrophytes was highest in the summer, followed by spring and winter seasons. Among emergents, Renuculus letus, Bidens pilosa, Bidens cirnua and Galium hertifolia were the most dominant in the summer and the spring seasons, and Myriophyllum verticillatum, in the winter season. After emergents, the next highest IVI values were those of submerged species in the winter and spring, followed by rooted floating - leaf type species in the winter and spring. Among the emergent species, Myriophyllum verticillatum was highly dominant throughout the year. The highest IVI values for this species were found in the winter (12.94), followed by the summer (7.30) and the spring season (6.98). Among the submerged species, Chara delicatula was highly dominant. The maximum IVI values for this species were recorded in the winter (12.70), followed by the spring (11.30) and the summer season (9.70). Consequently, the lowest IVI value for Chara delicatula was observed during the summer. Among the submerged species, Chara delicatula (IVI: 12.70) Hydrilla verticillata (IVI: 12.04) and Najas spp. (IVI: 10.70) were recorded to be the most dominant species throughout the year. Chara delicatula indicates its ability to adapt in miscellaneous conditions throughout the year. Among the growth forms, floating-leaved species were the least dominant in terms of average IVI value (19.81). The high growth of rooted floating-leaved species, especially Potamogeton nodosus was observed during the winter and spring seasons in this wetland. Annual average IVI values of emergent’s were found to be dominant (174.84), followed by the submerged (81.67), rooted floating-leaved species (23.82) and free-floating (19.81) macrophytes.
Statistical differences in the values of Importance value index (IVI) of macrophytes between seasons was also tested using ANOVA test. The difference between the seasons were found statically insignificant (one way ANOVA, emergents F=0.750, P=0.475, rooted floating - leaf type F=0.179, P=0.839, submerged F=2.56, P=0.095, free floating F=0.500, P=0.629).
Shannon-Weiner’s diversity index of freshwater macrophytes
The computation of Shannon-Weiner diversity index (H’) of macrophytes revealed that the species diversity was highest for emergents (2.48) in summer followed by the submerged, rooted floating leaf type and free-floating species respectively (Table 3). The highest species diversity for the entire community was found in the summer (3.77), as compared to 3.53 in the spring and 3.40 in the winter. The average value for the community as a whole was found to be 3.57 ± 0.15. Also, in a particular season, the diversity of different growth forms varied (Table 3) (Figure 5). For example, in summer season, the highest diversity was found for emergents (2.48) followed by submerged (0.80), rooted floating-leaf type (0.26) and free floating (0.23). The variation of diversity among different growth forms with respect to seasons and contribution of growth forms to diversity in different seasons is shown diagrammatically in (Figure 5). The highest species diversity index (3.77) for the entire community was found in the summer, as compared to the winter (3.40) and the spring (3.53). Thus, it has been revealed that there is a definite indication of significant seasonal change in the macrophytic community of the littoral zone of the Dodi Tal.
Growth Forms of Species |
Shannon - Weiner’ Index of Species Diversity (H) |
|||
Summer |
Winter |
Spring |
|
|
Emergent |
2.480 |
1.919 |
1.908 |
2.102 ± 0.27 |
Rooted - floating leaf type |
0.261 |
0.256 |
0.314 |
0.277 ± 0.03 |
Submerged |
0.807 |
1.032 |
0.915 |
0.918 ± 0.09 |
Free - Floating |
0.230 |
0.202 |
0.294 |
0.242 ± 0.04 |
Total community value (Dodi Tal) |
3.779 |
3.408 |
3.537 |
3.575 ± 0.15 |
Table 3 Seasonal variations in Shannon Weiner species diversity index (H’) value in different growth forms of macrophytes of Dodi Tal.
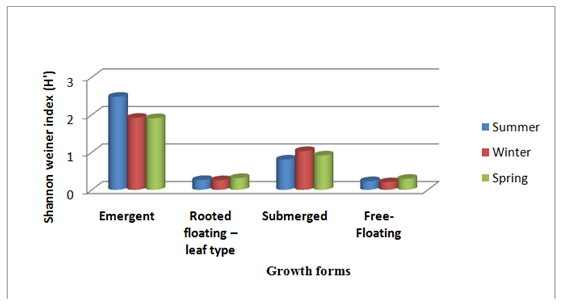
Figure 5 Seasonal variation in Shannon Weiner diversity index (H’) in different growth forms (emergent, rooted, submerged and free floating) macrophytes dwelling Dodi Tal.
Pielou’s Evenness index of freshwater macrophytes
The data on Pielou’s evenness calculated for macrophytes dwelling in Dodi Tal have been presented in Table 4. It was found to be maximum (J’=0.9949) in spring season and minimum (J’=0.9927) in winter season with highest species number (S=45) recorded in summer season. Pielou’s evenness index response depends on the manner in which the community changes. In this context evenness in Dodi Tal, there was no considerable change in the community of all the sampling sites.
Season |
Pielou’s Evenness Index (J’) |
||
S |
H’ |
J’ |
|
Summer |
45 |
3.779 |
0.9929 |
Winter |
31 |
3.408 |
0.9927 |
Springs |
35 |
3.537 |
0.9949 |
Table 4 Seasonal variations in Pielou’s Evenness index (J’) calculated for macrophytes of Dodi Tal.
S: Species Number; H': Shannon-Wiener Index; J': Pielou’s Evenness
The quantitative seasonal analysis of the macrophytic composition of the littoral zone of the high altitude lake may provide baseline information for formulating conservation and management strategies for this high altitude lake of Garhwal Himalaya. The quantity and diversity of macrophytes are the important parameters for assessing the health of the ecosystem. Influx of tourists can be regulated in different seasons for managing the integrity of the ecosystem. Reduction in the growth of macrophytes can be minimized it will improve the health of the ecosystem.
Emergent were the most dominant form throughout the year on the basis of IVI value. Myriophyllum verticillatum was highly dominant throughout the year. The highest IVI values for this species was found in the winter (12.94), followed by the summer (7.30) and the spring season (6.98). Higher growth of Myriophyllum verticillatum in the winter than in the summer may be due to sedimentation, snow melting, and human interference (highly nutrient enrichment present in inlet site 1 of this lake. The increasing abundance of emergents covering extensive areas in lake littoral zone of the wetland may be due to water level fluctuations accompanied with decreasing depth and nutrient enrichment mainly by allochthonous material by the way of sedimentation.34 This water milfoil species, Myriophyllum verticillatum is a good water oxygenator and also ideal in providing protection and respiration for fish spawn. Natural competition with other invasive aquatic plants has been the main control. So, we found that this species is very important for the purification of high altitude lake, Dodi Tal. This can be attributed to the emergent high tolerance for fluctuation of water level.35
After emergents, the next highest IVI values were those of submerged species in the winter and spring, followed by rooted floating - leaf type species in the winter and spring seasons. Among the submerged species, Chara delicatula was highly dominant. The high IVI values for this species were recorded in the winter (12.70), followed by the spring (11.30) and the summer season (9.70). During the summer, Chara delicatula was found to flow with water from the wetland due to seasonally high water levels and snow melting water including improper sanitation around the wetland. Consequently, the lowest IVI value for Chara delicatula was observed during the summer. No record of Chara delicatula has been found in high altitude lakes of The Himalayas. Its current dominance may be attributed to its invasive nature and also its preference for highly disturbed and stagnant water at temple site. Chara delicatula is found growing in the very clean and hard water lakes. Sometimes called musk grass, because they give off a musky or shunk- like odour. It is also a wetland indicator. The dynamic year-round growth of Chara delicatula indicates its ability to adapt in miscellaneous conditions. Crawfish (freshwater crustaceans) herbivory may select against perennial macrophytes and promote growth of pioneering plants like Chara.36 The factors like biotic interferences through local and tourist influx of nutrient rich run off not only affect the diversity of macrophytic species growing in these lakes, but also the entire biodiversity of the lacustrine ecosystem.14
The silt load and mixing of allochthonous material in the Dodi Tal were due to continuous landslides and runoff carrying a considerable silt, organic matter and litter from the catchment area. The dense growth of Hydrilla verticillata in the winter season can be attributed to the high growth potential of this species in sedimentation prone areas site 1 (inlet) of Dodi Tal. Sediment slope and texture also influence growth, distribution and occurrence of the macrophytes species is a particular water body.37,38 The dense growth of rooted floating-leaved species, especially Potamogeton nodosus in the winter and spring seasons, may be attributed to better adaptability of the rooted floating-leaved species to the stresses of water level fluctuations, to the tearing action of water turbulence, and to turbidity of water.39 Some species occurred across a wide range of lake plant richness, whereas, some species appeared to be good indicators of high richness like rooted floating - leaf type.40 The dense growth of free-floating and rooted floating-leaved species prevented colonization of submerged species in the summer and the winter seasons.41 Free floating macrophytes with significant dominant species, Lemna sp. and Salvinia natans. Lemna spp. are well known for its high productivity in littoral zones and high protein content in temperate climates.42
On the basis of annual average IVI values, it was found that emergents were dominant (174.84), followed by the submerged (81.67), rooted floating-leaved species (23.82) and free-floating (19.81) in Dodi Tal. Species diversity was highest for the emergents (2.480) in summer followed by the submerged, rooted floating leaf type and free-floating species respectively. The same seasonal trend was reported by Van der Valk & Davis34 and Handoo & Kaul43 in their studies. Species diversity is a useful parameter for the comparison of communities under the influence of biotic disturbance or to know the state of succession and stability in the community.44 The seasonal variations in miscellaneous growth forms may cause the variations in the species diversity. The diversity index (H’) for macrophyte ranged from 3.40-3.77 in Dodi Tal. These are comparable with those reported by Burlakoti et al.2 (3.17-4.17) in Beeshazar, Chitwan, Nepal; Sharma14 (0.51-1.50) in Jammu and Kashmir, India; Sarmah et al.12 (2.51-3.21) in Monrikhaboloobeel wetland of river Subansiri, Assam; Sharma et al.44 (2.70-1.77), India and Biswas et al.45 (2.30-0.86) Chhariganga oxbow lake in Nadia district, West Bengal, India. All these studies have been conducted in different parts of Asia.
The present quantitative analysis of macrophytes shows a definite indication of significant changes in the macrophytic community of the Dodi Tal. The most significant change in macrophytic community may be due to heavy siltation in the catchment area of the lake is due to frequent landslides at the tourist’s activities depending on their influx in different seasons. The silt raising the lake thick layer brings in large quantities of nutrients in to the lake. In oligotrophic and mesotrophic lakes, nutrient loading increases tolerant aquatic macrophyte species abundance.46 Another reason for increasing sedimentation is the climatic change due to global warming ensuing in increased melting of glaciers, there by bringing in more and more sediments into the lake as the main source of water to the lake is neighboring glaciers.34
The abundant growth of the macrophytes in the littoral zone of the Dodi Tal wetland reveals the dynamic nature of the wetland. The dominance of emergents among other growth forms (as shown by IVI dimensions) indicates the disturbance caused by the various anthropogenic activities of the tourists in the riparian zone of the wetland. Based on the above results, it can be concluded that the high altitude wetland, Dodi Tal, showed high diversity of macrophytes during summer season. The obtained Shannon-Weaver diversity index value (H’=3.57) indicates rich species diversity in Dodi Tal lake. The dominance of Chara delicatula indicates its invasive nature of this macrophytes. The fact that emergents have the highest species diversity and lowest free floating species signifies the increasing richness in species with decreasing water level. Therefore, it is suggested that Chara delicatula should be removed immediately from this high altitude wetland ecosystem, so that it should not further deteriote the health of the ecosystem. For overall conservation and management of this high altitude wetland, the influx of tourists should be regulated in various seasons for minimizing and arresting the growth of the macrophytes, consequently imporving the health of the Dodi Tal.47
One of the authors (Sushma Singh) is thankful to the University Grants Commission, New Delhi and H.N.B Garhwal University (A Central University) Srinagar - Gharwal, Uttarakhand for providing her fellowship during the period of study.
The authors declare that there is no conflict of interests to declare regarding the publication of this paper.

©2017 Sharma, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.