eISSN: 2378-315X


Research Article Volume 11 Issue 2
1BVBGR-LR11ES31, University of 5 Manouba, Tunisia
2Faculty of Sciences of Tunis, University of Tunis El Manar, Tunisia
3APVA-LR16ES20, University of Sfax, Tunisia
Correspondence: Mohamed Neifar, Department of biotechnology, Tunisia
Received: April 21, 2022 | Published: June 10, 2022
Citation: Souii A, Gorrab A, Hammami K, et al. Statistical optimization of cellulase production by halophilic strains isolated from Tunisian Sebkhas using the renewable marine biomass waste, Posidonia oceanica, as a cellulosic substrate. Biom Biostat Int J. 2022;11(2):65-70. DOI: 10.15406/bbij.2022.11.00356
Bio-based ethanol production from enzymatic lignocellulosic biomass degradation serves an efficient technology to combat the problem of depleting fossil fuels. High cost of the enzyme is the major obstacle in preventing the commercialization of this process. Thus, main objective of the present study was to optimize composition of culture parameters for enhancing cellulase production by two halophilic strains Oceanobacillus picture E50 and Bacillus vallismortis J77 using Posidonia oceanica waste (POW) as a substrate. A Plackett-Burman design was conducted to study and evaluate the weight of eight cultural variables on bacterial cellulase production. The optimal culture conditions for maximum enzyme yield were developed by maintaining the variables of POW concentration, NaCl concentration, POW liquor, bacterial strain and inoculum size at their higher levels, while keeping substrate particle size, glucose concentration and incubation time at their lower levels. The highest cellulase production in terms of CMCase activity was 950 U/L following this optimization process. Thus, the results obtained in this study demonstrated the potential of utilizing POW as a substrate for enhanced cellulase production by halophilic bacteria. B. vallismortis J77 produced the highest cellulase activity and therefore could be of great interest for saccharification of Posidonia oceanica biomass for biofuel and biorefinery applications.
Keywords: carboxymethylcellulase, Posidonia oceanica, Oceanobacillus picture, Bacillus vallismortis, fermentation parameter optimization, plackett–burman design
Microbial cellulases have been studied mainly with the aim of converting agricultural biomass into useful products. Among the classes of cellulases, endoglucanases often measured as carboxymethylcellulases (CMCases) have been used predominantly in the cellulosic biomass saccharification process for bioethanol production. Commercial cellulases are mainly produced by fungi belonging to Aspergillus & Trichoderma genera. Many bacteria have been also gained attention as robust and versatile biocatalyst producers because of their high growth rate, the presence of multi-enzyme complexes and their stability at extreme conditions. Among these, Bacillus strains have been revealed to be the most potent hydrolytic extremozymes producers.1–4 Extremophilic microorganisms capable of utilizing cheap agricultural wastes under harsh conditions could be exploited as an economic alternative to existing production processes.
In general, many quantitative and qualitative variables are involved in the fermentative process for enzyme production, and for this reason statistical experimental designs have been widely used for screening experiments or for optimization studies.5 The results generated by the statistical designs allow to reduce the experimental efforts without losing reliability. They are better exploited than those obtained by the traditional one variable at a time (OVAT) method. A popular class of statistical experimental designs is the Plackett-Burman design which offers a fast and efficient tool to identify the important factors, thereby, saving time and maintaining convincing information on each system variable.6,7 Statistical optimization of enzyme production by mesophilic microbial strains has been the subject of intensive studies. However, results on modelling and optimizing microbial extremozymes recovery for bioeconomical processes are very scarce in the literature and should be helpful to understand the true potentialities of green biocatalysts in its industrial applications.4,8,9
In this study, a Plackett–Burman statistical experimental design was applied to optimize CMCase production by two extremophilic bacteria Oceanobacillus picture E50 and Bacillus vallismortis J77. These halophilic and cellulolytic bacteria have been isolated previously from desertic saline environments in Tunisia.10 To the best of our knowledge, this is the first report designing Posidonia oceanica saline waste (POW)-based medium for cellulase production under submerged cultivation of bacterial halophiles. The residues of P. Oceanica, a dominant seagrass in the Mediterranean Sea, are continually removed to clean touristic beaches.11,12 Their chemical composition have been widely investigated. The dry POW were composed principally of lignin and holocellulose. Holocellulose represents the total seagrass carbohydrate content and has been calculated to be 61.8% and cellulose contributes 40%.13 These data make POW a renewable cellulose source14 and a potential feedstock for bioethanol production.15
Microorganisms and substrates: Halophilic (up to 15% NaCl) bacteria, Oceanobacillus picture E50 and Bacillus vallismortis J77 were isolated from coastal Sebkhas in Tunisia, and the strains were maintained on nutrient agar slants.10 POW was collected from a Tunisian beach in sterile polythene bags. It was dried at 50°C in hot air oven for 48 h, ground by using a conventional grinder and stored in the dark at +4 °C. A known weight of dried materials were blended by a mixer with equal volumes of water, filtered, sterilized by autoclaving. The whole sterilized blend was used as a PW liquor. Carboxymethylcellulose (CMC) was purchased from Sigma-Aldrich (St. Louis, USA).
CMCase production, assay and zymography: CMCase activities of selected bacteria were firstly revealed on CMC agar media (1% w/v). To visualize the hydrolysis zone after bacterial incubation at 50 °C for 3 days, the plates were flooded with an aqueous solution of 0.1% Congo red for 30 min and washed with 1 M NaCl.4 Culture medium used for cellulase production contained (g/L): Yeast Extract 0.25 g/L; MnSO4·7H2O 0.5 g/L; (NH4)2SO4 0.25 g/L and KCl 0.5 g/L.16 After sterilization and cooling, the flasks were inoculated with overnight grown bacterial culture. At the end of the fermentation period, the culture broth was centrifuged for 10 min at 10,000 rpm to obtain the crude extract, which served as enzyme source.10 Enzyme activity was determined by the reducing sugars produced in a modified dinitrosalicylic acid (DNS) method17 using glucose as the standard. The composition of DNS reagent was as follows: 3.2 g sodium hydroxide, 2 g 3,5-dinitrosalicylic acid and 60 g potassium sodium tartrate in 200 mL H2O. The assay was performed as described previously by Souii et al.4 One unit of cellulase activity was expressed as the quantity of enzyme, required to release 1 µmol of glucose per min per mL under standard assay conditions. For the analysis of proteins secreted by the bacterial strains, polyacrylamide gel electrophoresis18 under native (Native-PAGE) conditions were performed with zymograms to determine cellulase activity. The gels were stained with 0.2% Congo red and washed with 1M NaCl until bands appeared to reveal the cellulase activity, according to previously described methods.3,9,19
Statistical design experiments for enzyme production optimization: Developed in 1946 by statisticians Robin L. Plackett and J.P. Burman, Plackett-Burman experimental design is an efficient tool to screen a large number of parameters and identify the active factors using as a relative small number of experiments.5 In this study, the Plackett–Burman experimental design was used for screening and optimizing of cultural variables having significant effect on CMCase production. Plackett–Burman experimental design is a two-level fractional design that follows first-order polynomial model:
With: Y response variable (CMCase activity), model intercept, linear coefficient, and level of independent variable, is an error term.
Totally eight culture factors, potentially influencing the cellulase production, have been examined at two levels.20 This experimental design comprised of a total of 24 sets of experiments with permutation combination of different factors and their levels. The average enzyme production in each experiment was considered as the response. Each experiment was conducted in duplicate to assess reproducibility. The characteristics of Plackett–Burman statistical experimental design are given in Tables 1 & 2. To determine the weight of a factor, we calculate the difference between the average of the responses obtained at one level and the average obtained at another level of the factor, or the general average of all the responses of the experimental design. From the 24 runs, the determination of model coefficients was calculated using the least square method. The results were exploited by an analysis of variance and graphical analyses. Analysis of variance (ANOVA) is intended to distinguish the factors that have a statistically significant influence on the average level of the response. The total variance observed at the level of a response is the sum of the partial variations attributable to the factors studied (controlled) and a residual variation due to experimental error and uncontrolled or uncontrollable factors. Each variation is estimated by a sum of the deviations from the mean (SS) with a certain number of degrees of freedom. Statistical tests of significance are conducted using the Fisher test: the Fexp value of the ratio of the variance of a factor (calculated with n1 ddl) to the residual variance (calculated with n2 ddl) is compared to the Fisher critical value F0.05 (n1, n2). The effect is considered statistically significant at the 95% confidence level if Fexp > F0.05 (n1, n2). The quality of fitting the first-order polynomial was expressed by the coefficient of regression R2, which measures the proportion of total variation about the mean response explained by the regression. For graphical analysis of the experimental design model, the averages obtained at each factor level are plotted. The magnitude of the effect of the different factors can also be examined by means of a graph of the differential effects of the factors. To do this, the incremental effects calculated by considering two levels of the variable are plotted. The significant incremental effects are those that exceed the limits of the confidence interval (two vertical dashed lines). In this work, the generation and the data treatment of the Plackett–Burman experimental design are performed using the experimental design software NEMRODW.21
|
Coded variables |
Factors (Units) |
Coded levels |
levels |
|
X1 |
POWconc. (%) |
-1 |
0.5 |
|
+1 |
1 |
||
|
X2 |
Substrate particle size (mm) |
-1 |
0.5 |
|
+1 |
5 |
||
|
X3 |
NaCl conc. (mM) |
-1 |
0 |
|
+1 |
500 |
||
|
X4 |
POW liquor (%) |
-1 |
0 |
|
+1 |
10 |
||
|
X5 |
Glucose conc. (%) |
-1 |
0 |
|
+1 |
0.2 |
||
|
X6 |
Bacterial strain |
-1 |
O. picture E50 |
|
+1 |
B. vallismortis J77 |
||
|
X7 |
Inoculum size (%) |
-1 |
2 |
|
+1 |
4 |
||
|
X8 |
Incubation Time (days) |
-1 |
4 |
|
+1 |
8 |
Table 1 Experimental domain for the Plackett-Burman experimental design
|
N° exp |
PW conc. (%) |
PW particle size |
NaCl conc. (mM) |
PW liquor (%) |
Glucose conc. (%) |
Bacterial strain |
Inoculum size (%) |
Incubation time (d) |
Experimental cellulase activity (U/L) |
Theoretical cellulase activity (U/L) |
|
1 |
1 |
5 |
0 |
10 |
0.2 |
BV J77 |
2 |
4 |
422.000 |
417.354 |
|
2 |
1 |
5 |
0 |
10 |
0.2 |
BV J77 |
2 |
4 |
397.000 |
417.354 |
|
3 |
0.5 |
5 |
500 |
0 |
0.2 |
BV J77 |
4 |
4 |
87.500 |
76.471 |
|
4 |
0.5 |
5 |
500 |
0 |
0.2 |
BV J77 |
4 |
4 |
62.700 |
76.471 |
|
5 |
1 |
0.5 |
500 |
10 |
0 |
BV J77 |
4 |
8 |
739.200 |
715.396 |
|
6 |
1 |
0.5 |
500 |
10 |
0 |
BV J77 |
4 |
8 |
710.600 |
715.396 |
|
7 |
0.5 |
5 |
0 |
10 |
0.2 |
OP E50 |
4 |
8 |
18.200 |
7.171 |
|
8 |
0.5 |
5 |
0 |
10 |
0.2 |
OP E50 |
4 |
8 |
33.600 |
7.171 |
|
9 |
0.5 |
0.5 |
500 |
0 |
0.2 |
BV J77 |
2 |
8 |
68.700 |
78.529 |
|
10 |
0.5 |
0.5 |
500 |
0 |
0.2 |
BV J77 |
2 |
8 |
91.100 |
78.529 |
|
11 |
0.5 |
0.5 |
0 |
10 |
0 |
BV J77 |
4 |
4 |
440.000 |
444.204 |
|
12 |
0.5 |
0.5 |
0 |
10 |
0 |
BV J77 |
4 |
4 |
429.400 |
444.204 |
|
13 |
1 |
0.5 |
0 |
0 |
0.2 |
OP E50 |
4 |
8 |
148.700 |
157.179 |
|
14 |
1 |
0.5 |
0 |
0 |
0.2 |
OP E50 |
4 |
8 |
128.200 |
157.179 |
|
15 |
1 |
5 |
0 |
0 |
0 |
BV J77 |
2 |
8 |
423.600 |
393.696 |
|
16 |
1 |
5 |
0 |
0 |
0 |
BV J77 |
2 |
8 |
379.500 |
393.696 |
|
17 |
1 |
5 |
500 |
0 |
0 |
OP E50 |
4 |
4 |
340.700 |
325.279 |
|
18 |
1 |
5 |
500 |
0 |
0 |
OP E50 |
4 |
4 |
312.600 |
325.279 |
|
19 |
0.5 |
5 |
500 |
10 |
0 |
OP E50 |
2 |
8 |
148.700 |
177.329 |
|
20 |
0.5 |
5 |
500 |
10 |
0 |
OP E50 |
2 |
8 |
168.500 |
177.329 |
|
21 |
1 |
0.5 |
500 |
10 |
0.2 |
OP E50 |
2 |
4 |
339.100 |
350.996 |
|
22 |
1 |
0.5 |
500 |
10 |
0.2 |
OP E50 |
2 |
4 |
378.600 |
350.996 |
|
23 |
0.5 |
0.5 |
0 |
0 |
0 |
OP E50 |
2 |
4 |
38.700 |
56.146 |
|
24 |
0.5 |
0.5 |
0 |
0 |
0 |
OP E50 |
2 |
4 |
92.600 |
56.146 |
Table 2 Experimental design of the enzyme production process with the responses recorded for each experiment
The growing demand for renewable energy has focused worldwide attention on the use and valorization of lignocellulosic biomass, particularly agricultural wastes and industrial byproducts.22 These lignocellulosic materials have a high organic content and their disposal poses both economic and environmental problems. On the other hand, their main carbohydrate components such as cellulose, hemicellulose and starch can be used by many microorganisms as carbon and energy sources, producing high added-value products such as ligninolytic, hemicellulolytic and cellulolytic enzymes.4,8,9,23 In this context, this work describes the potential use of POW as substrate for microbial cellulase production and this constitutes an environmentally friendly method for the valorisation of this waste.
The two bacterial cultures, O. picture E50 and B. vallismortis J77, isolated from Tunisian coastal Sebkhas were firstly screened for cellulase production on CMC agar plate. After incubation, they showed prominent zone of clearance around the colonies indicating extracellular cellulolytic activity (Figure 1a). Zymogram analysis was also performed to detect extracellular enzymes active toward the CMC substrate. The zymogram profile revealed three and four bands with CMCase activities for O. picture E50 and B. vallismortis J77, respectively which supposed that different endoglucanases were produced by these strains (Figure 1b). The obtained enzymatic profiles are in line with that reported by Caf et al.24 but totally different with that described by Potprommane et al.9 who detected the presence of the single zymogram band with CMCase activity produced by Geobacillus sp. HTA426. The biochemical characterization of the crude enzymatic extracts of the strains E50 and J77 showed high stabilities of cellulases against temperature, pH and salinity (data not shown). These results are in agreement with those reported for other Bacillus strains with high bioconversion potential.25–33
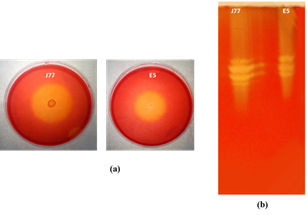
Figure 1 Detection of CMCase activities from Oceanobacillus picture E50 and Bacillus vallismortis J77 on CMC agar plate and fermentation liquid medium (a)Plate assay for detection of CMCase activity. CMC agar plates were stained with Congo Red and enzyme activity is detected by the presence of a halo around the colony; (b)Zymogram validating cellulolytic activity on 1% CMC supplemented gel stained with 0.1% (w/v) congo red after destaining with 1M NaCl.
After enzyme detection on agar plates and by zymography, eight nutritional factors including POW concentration, substrate particle size, NaCl concentration, POW liquor, Glucose concentration, bacterial strain, inoculum size and incubation time were selected in the present investigation as the key factors affecting the cellulase production by selected halotolerant bacteria. The levels of factors were selected by taking into account the operating experimental limits, and the previous studies.34–36 The domain and matrix characteristics of Plackett-Burman experimental design used for the optimization of cellulase production were presented in Tables 1&2, respectively. As shown in Table 2, the experimental and predicted values of CMCase activity match quite well. The overall regression coefficient for the Plackett–Burman design is . It implies that 99.1 % of the variability in the enzyme production response could be explained by the experimental model, which proves that it is good and very reliable. According to the table of the analysis of variance (Table 3), we can conclude that the regression explains the studied phenomenon very well since the significance of the risk (p value< 0.001) is lower than 0.05. The statistical effects of the studied variables are shown in Table 4. Each variable who has a high coefficient is delimited by the vertical lines (Figure 2) that corresponds to the 95%¨limit indicating statistical significance.37,38 As seen from Table 4, this condition is satisfied by all variables. These results are clearer on the graph of the factors’ effects (Figure 2). The individual and cumulative effects of various culture parameters on enzyme production can be discussed from the Pareto chart illustrated by Figure 3. Simultaneous statistical and graphical analysis clearly showed that POW concentration, substrate particle size, glucose concentration, and incubation time had negative effects, while all other variable displayed positive effects on the response. The POW concentration was the most important factor inducing the enzyme production with a coefficient of 126.67. This parameter alone contributes 39.66% of the variability in the studied response. Cellulase production by the strains O. picture E50 and B. vallismortis J77 enhanced with the increase of substrate concentration and the derease of substrate particle size. Similar patterns were obtained by many studies.16,39–48 The bacterial strain used for enzyme production is the second factor influencing the response with a coefficient of 87.63 and a contribution of 18.98% in yield variability. B. vallismortis J77 expressed high CMCase activity compared with O. picture E50. The high enzyme yield of B. vallismortis suggest its potential for enzymatic saccharification of POW for bio-fuel production.41 The experimental design results indicated also that enzymatic activity increases with POW liquor concentration. This could be explained by the richness of the P. oceanica extract in nutrients that promote bacterial growth and cellulase production.40 The validation of Plackett-Burman experimental design model was performed by carrying out experiments under optimized conditions: POW concentration (1%); substrate particle size (1mm); NaCl conc. (500mM); POW liquor (10%); glucose concontration (0%); bacterial strain (B. vallismortis J77); inoculum size (4%) and incubation time (4 days) established by the regression model. A maximum CMCase production of 950 IU/L was obtained from experiments which was very close to the theoretical yield (942 IU/L) predicted by the regression model, providing the validity of the Plackett–Burman model.
|
Source of variation |
Sum of squares |
Degrees of freedom |
Mean square |
Ratio |
Significance |
|
Regression |
970927 |
8 |
121365 |
214.4578 |
*** |
|
Residuals |
8488.79 |
15 |
565.92 |
|
|
|
Lack of fit |
3028.07 |
3 |
1009.35 |
2.2181 |
13.80% |
|
Error |
5460.73 |
12 |
455.06 |
|
|
|
Total |
979416 |
23 |
|
|
|
Table 3 Analysis of variance for the fitted model of the Plackett-Burman design
***Significant at the level 99.9%.
|
Name |
Coefficient |
Ecart-Type |
t.exp. |
Signif. % |
|
b0 |
266.646 |
4.856 |
54.91 |
*** |
|
b1 |
126.671 |
4.856 |
26.09 |
*** |
|
b2 |
-33.763 |
4.856 |
-6.95 |
*** |
|
b3 |
20.688 |
4.856 |
4.26 |
*** |
|
b4 |
85.429 |
4.856 |
17.59 |
*** |
|
b5 |
-85.363 |
4.856 |
-17.58 |
*** |
|
b6 |
87.629 |
4.856 |
18.05 |
*** |
|
b7 |
20.971 |
4.856 |
4.32 |
*** |
|
b8 |
-11.763 |
4.856 |
-2.42 |
* |
Table 4 Estimated regression coefficients for the Plackett and Burman design and their significations
* Statistically significant for a level of confidence of 95%;
*** Statistically significant for a level of confidence of 99.9%
The present study shows the potential of valorizing Posidonia oceanica waste as a new inducer substrate for the production of halostable cellulases by extremely halophilic strains Oceanobacillus picture E50 and Bacillus vallismortis J77. The statistical experimental Plackett-Burman design applied for the optimization of enzyme production revealed that most critical process parameters influencing the enzyme production were the POW concentration and the bacterial strain producer. It was observed that the screening of significant culture conditions reduced the number of experiments leading to a considerable reduction in process operating costs. In the light of these results, we will increase our efforts to : (i) purify and biochemically characterize the bacterial extremozymes to apparent homogeneity to better elucidate the mode of action of these biocatalysts, (ii) predict 3D structural models that can be used as basal structures to improve the catalytic efficiency, (iii) immobilize bacterial cellulases as a strategy for improving enzyme activity and stability with increasing reuse cycles and (iv) demonstrate the bacterial extremozymes-based technology at a pilot scale for bioconversion of cellulosic wastes into bioethanol, and render them more attractive in a sustainable bioeconomy perspective.49,50
The authors acknowledge financial support from the Young Teacher-Researchers Program granted to Dr. Mohamed Neifar in the ambit of his project EC06-07 (2018–2020) “Bioconversion of cellulosic material from Posidonia oceanica residues into bioethanol”51,52 and the Tunisian Ministry of Higher Education and Scientific Research in the ambit of the laboratory project LR11ES31.
The authors declare no conflicts of interest.

©2022 Souii, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
2 7