Advances in
eISSN: 2572-8490


Research Article Volume 5 Issue 2
1Department of Biotechnology & Bioinformatics, California State University, USA
2Department of Biomedical Engineering, University of California, USA
Correspondence: Bill Tawil, Biotechnology & Bioinformatics Master’s Program, California State University, 420 Westwood Plaza, Room 5121, Engineering VPO Box 951600, Los Angeles, CA 90095–1600, USA
Received: May 18, 2019 | Published: June 26, 2019
Citation: Jennifer T, Ploy U, Bill T. The behavior of skeletal muscle cells in various water types, ibuprofen and three-dimensional fibrin constructs. Adv Tissue Eng Regen Med Open Access. 2019;5(2):74-83. DOI: 10.15406/atroa.2019.05.00103
An increasingly popular health trend suggests that an alkaline diet will provide numerous benefits to the human body.1 In this study, the effects of water from different brands and sources, including one high in alkalinity, were tested to observe the cell proliferation of both human foreskin fibroblast (HFF-1) and rat skeletal muscle cells (L6). It was found that a 1:1 ratio of alkaline water to media did not significantly impact the characteristics (initial adhesion, proliferation, or morphology) of neither fibroblast nor muscle cells when compared to spring water and local water. The addition of water, regardless of its source, decreased the proliferation of HFF-1 cells but did not impact the proliferation of L6 cells. In further analyzing the behavior of muscle cells various concentrations of ibuprofen were used to observe changes in initial adhesion and proliferation. It was found that a concentration greater than 0.05mg/mL of ibuprofen inhibited L6 cell proliferation while a concentration less than 0.05mg/mL had no effect. Examination of L6 proliferation/migration in three-dimensional fibrin beads compared to 3D printed constructs showed that L6 cells in fibrin beads migrated more with a lower fibrinogen concentration (10mg/mL) and cells in fibrin beads were distributed more uniformly than 3D printed constructs. Various water sources did not impact the characteristics of L6 cells while high concentrations of ibuprofen were found to inhibit cellular adhesion and proliferation. Furthermore, fibrin beads were found to be an optimal 3D environment for L6 cell migration.
A diet that is high in alkaline is believed to play a significant role in improving the health of the human body.1 Studies claim that there is a wide range of benefits resulting from this kind of diet, specifically supporting the improvement in muscle mass.2 A condition called metabolic acidosis is commonly seen in aging adults and occurs when the body is unable to remove the acidic residues that are normally metabolized from foods containing grains or proteins.3 This condition would result in a more acidic environment of the body and is said to lead to deterioration of muscle mass which is detrimental to bone health.3 Eating more fruits and vegetables that the body will metabolize into alkaline residues, or even drinking liquids high in alkaline could help provide a healthy balance to the body’s pH level in aging adults.3 To test out this theory, a popular brand of water, Essentia is advertised to provide these benefits due to the high alkalinity of their water. It is made through a proprietary process that makes it 99.9% pure, infuses electrolytes, and removes bitter tasting acidic ions leaving the water at a pH 9.5 or higher.4 Essentials was tested against Arrowhead, a standard spring water used for drinking, and also local sink water. Both HFF-1 fibroblast cells and L6 muscle cells were used to compare trends in cellular adhesion and proliferation between the two cell types. The initial theory suggests that the Essentia water will promote a significantly increased level of adhesion and proliferation in the L6 muscle cells compared to other water types. The same effects would apply to the HFF-1 cells based on the theory that an alkaline environment is generally beneficial for the body. HFF-1 cells were selected as it is a common cell line studied to represent nonmuscle cells. To further explore the characteristics of muscle cells, various concentrations of ibuprofen were also used in this study. Ibuprofen is a commonly used over-the-counter drug that is taken to relieve muscle aches and pains. A previous study had performed 24-hour viability assays to analyze the impact of ibuprofen on rat Achilles tendon cells using .05, .1, .2, and 4mg/mL concentrations of ibuprofen.5 It was indicated that increasing concentrations of ibuprofen was directly related to the inhibition of Rb protein phosphorylation which is a protein that plays a critical role in the eukaryotic cell cycle for cell proliferation, and that anti-inflammatory drugs like ibuprofen actually inhibit migration and proliferation of some muscle cell types.5 To further understand the impact of ibuprofen on muscle cells, 2D proliferation assays were performed using L6 muscle cells in .05mg/mL and .025mg/mL of ibuprofen over a 7-day period. Additional experiments were conducted to observe optimal 3D environments for L6 cells. Future applications of 3D constructs with various water types and concentrations of ibuprofen may provide additional insight into the behavior of L6 cells when exposed to these environments.
HFF-1 and L6 cell culture
HFF-1 cells (ATCC, Manassas, VA, USA) and L6 cells (ATCC, Manassas, VA, USA) were cultured in T75 flasks (Thermo Scientific TM, Waltham, MA, USA) and stored in an incubator (Thermo Scientific TM, Waltham, MA, USA) at 37 and 5% CO2. After cells were determined to be the appropriate confluency, using a phase contrast microscope CKX41 (Olympus, Shinjuku, Tokyo, Japan), the media was removed from the cells. Phosphate buffer saline (PBS) (GE Healthcare, Chicago, IL, USA) was used to wash the flask and removed. An addition of 5mL of 0.25% trypsin (GE Healthcare, Chicago, IL, USA) was added to the flask and left to incubate at room temperature for 10 minutes. The phase contrast microscope was used to ensure the cells had completely detached from the flask. An addition of 5 mL of HFF-1 media or L6 media was added to the flask to inhibit trypsin activity. The solution of 10 mL of cells and media was transferred to a 15 mL conical tube (Fisherbrand, Waltham, MA, USA) and spun down in a centrifuge (Eppendorf AG, Hamburg, Germany) at 200 rpm, at 5, for 5 minutes. The supernatant was carefully removed leaving a small white pellet. Resuspension of the cells was performed by adding 1 mL of media to the tube and 2-20µL of the solution was added to a Cellometer slide (Nexcelom Bioscience, Lawrence, MA, USA). The Cellometer Auto T4 (Nexcelom Bioscience, Lawrence, MA, USA) program was used to determine the averaged cell concentration between the two readings. To determine whether plates would be coated with fibronectin or collagen throughout the study, an experiment was performed using three 24-well plates (Corning Incorporated, Corning, NY, USA) coated with 5µg/mL and 10µg/mL of collagen (Sigma-Aldrich, St. Louis, MO, USA) and 5µg/mL and 10µg/mL of fibronectin (Sigma-Aldrich, St. Louis, MO, USA). 5,000 cells were seeded in each well with HFF-1 media and incubated at 37 and 5% CO2.
2D Cell proliferation assay
Various water types
Alkaline water (Essentia, Bothell, WA, USA) was obtained from Target in Camarillo, CA. Spring water (Arrowhead, Stamford, CT, USA) was obtained from Ralphs in Thousand Oaks, CA. Local water (sink water, Ventura, CA, USA) was obtained from the sink at California State University in Channel Islands in Camarillo. The pH (Micro Essential Laboratory, Brooklyn, NY, USA) was obtained of all water types to be around 7 and 8. All water was vacuum filtered with Nalgene™ 25mm Syringe Filters (Thermo Scientific TM, Waltham, MA, USA). HFF-1 and L6 media was treated with the various types of water at a 1:1 ratio of media to water. Three 24-well plates were coated with collagen at 10µg/mL. 10,000 cells were seeded per well with HFF-1 media or L6 media with appropriate water type, and incubated at 37°C and 5% CO2.
Various concentrations of Ibuprofen
Different concentrations of ibuprofen were obtained to determine the levels at which L6 cells are positively or negatively impacted during initial adhesion and proliferation. Liquid gel capsules of Ibuprofen (Target Corporation, Minneapolis, MN, USA) were obtained from Target in Camarillo. Ibuprofen capsule contained 200mg strength. The solubility of Ibuprofen in ethanol (Fisher Chemical, Fair Lawn, NJ, USA) was obtained from literature while the pH of the solution was observed to be around 7 and 8 at 200mg in 700µL of ethanol.6,7 Solution of Ibuprofen and ethanol was vacuum filtered and diluted appropriately to obtain various concentrations of 0.1mg/well and 0.05mg/well which is equivalent to 0.05mg/mL and .025mg/mL concentrations respectively. Ethanol was also vacuum filtered and mixed with media to obtain 3.5µL/well. Three 24-well plates were coated with collagen at 10µg/mL. 10,000 cells were seeded per well with L6 media with appropriate concentrations of Ibuprofen or ethanol, and incubated at 37°C and 5% CO2.
3D Printing and fibrin beads
L6 cells were observed in three dimensional environments to determine alternative and optimal conditions different from the 2D assays performed throughout the study. Preparation for 3D printing was performed by transferring approximately 2million L6 muscle cells to 3mL of ink mixture containing gelatin and alginate. The solution was poured into a 10mL plastic syringe which was placed into the canister of the 3D printer (Biobot, Philadelphia, PA, USA). The solution was extruded from the syringe onto a 6-well plate (Corning Incorporated, Corning, NY, USA) at a hydraulic pressure of 8 psi with a print speed of 6mm/sec to obtain .2mm thickness of approximately 6 layers of the ink material. The temperature of the canister was maintained at 37°C. In the 3D fibrin bead environment, 24-well plates were used to create 3D matrices in the wells containing 10mg/mL and 20mg/mL concentrations of fibrinogen following the addition of 20 units/mL of thrombin. L6 muscle cells were mixed with 40mg/mL of fibrinogen to deliver approximately 10,000 cells per bead. 10µL of the fibrinogen and cell mixture was added to the well creating a tiny droplet. Then, 10µL of 40 units/mL of thrombin was added to the same droplet to form the fibrin bead. Plates were incubated at 37°C and 5% CO2.
Statistical analysis
Data is presented as mean ± standard deviation (SD). A Paired Student t-test was used to determine statistical significance of observed differences between the study groups. A value of p<0.05 is considered statistically significant.
The effect of collagen and Fibronectin on HFF-1 cellular adhesion and proliferation
In this study we will be looking at the cellular adhesion and proliferation of HFF-1 cells on wells coated with 5µg/mL collagen, 10µg/mL collagen, 5µg/mL fibronectin, and 10µg/mL fibronectin to determine the best conditions for cellular proliferation. We will also be observed the cell morphology for any differences between each condition. Initial adhesion of HFF-1 cells on various concentrations of collagen and fibronectin at 5µg/mL or 10µg/mL were confirmed. The initial adhesion is observed in percent adhesion after one hour incubation. The greatest initial adhesion was observed in the condition with 5µg/mL fibronectin coated wells at 374% as compared to the lowest adhering condition, 10µg/mL collagen at 100%, as seen in Figure 1A. Cell proliferation is expressed in a percentage as compared to day 1 at 100%. Figure 1B shows that greatest proliferation was observed in 10µg/mL collagen, while 5µg/mL fibronectin showed the least proliferation. When concentration of both collagen and fibronectin increased, proliferation increased. On day 3 in all conditions, there was a decrease in proliferation as compared to day 1 and on day 7 proliferation decreased in 5µg/mL fibronectin. HFF-1 cells showed similar circular shape in all conditions as shown in Figure 1C. Highest cell density was observed in collagen at 5µg/mL. Day 3 showed elongated cells that spread evenly across the well. Day 7 showed similar confluency as day 3. This indicates that HFF-1 cells likely reached maximum confluency by day 3. Results show that 10µg/mL collagen coated wells showed highest cellular proliferation. All studies are performed on 10µg/mL collagen coated wells to ensure an environment ideal for proliferation. With an increasing trend of alkaline diets for health benefits, the addition of various types of water was studied to determine its effects on proliferation on 10µg/mL collagen coated wells.
The effect of various water types on HFF-1 and L6 cells
HFF-1
In this study we will be looking at the effects of various types of water, alkaline, spring, and local water, on cellular adhesion and proliferation of HFF-1 cells on wells coated with 10µg/mL collagen. We will also be observing the cell morphology for any differences between each condition. Initial adhesion of HFF-1 cells in various water types, alkaline water, spring water, and local water, on day 1 was analyzed. The greatest initial adhesion was observed in Figure 2A for alkaline water at 140% and least in spring water at 119%, while local water showed 121% adhesion as compared to 100% media. Spring water and local water were observed to have similar percent adhesion. This indicates that HFF-1 cells adhered to the wells successfully. Cell proliferation was expressed in a percentage as compared to day 1 at 100% as seen in Figure 2B. The greatest cell proliferation was observed in local water at 560%, followed by alkaline water at 394%, and lastly, in spring water at 377%. Proliferation in all water types was about 1-2-fold less than media proliferation rates overall, while proliferation between water types was not affected. Proliferation of HFF-1 cells in local water on day 3 increased by less than 1-fold, while day 7 as compared to day 3 increased by 3-fold. Alkaline water and spring water showed similar proliferation rates, with about a 1-fold increase from day 1 to day 3 and remaining steady by day 7. Morphology of HFF-1 cells on day 1 was present and well rounded as shown in Figure 2C. On day 3, HFF-1 cells in water showed slightly difference morphology, especially in width. Alkaline water showed small, thin, elongated cells, local water showed mid-length cells with slightly thicker width, and spring water showed thickest width in cells with similar elongation. By day 7, cell density increased in alkaline water and media significantly while cells were observed to elongate slightly more as compared to day 3. While alkaline water did not show any significant difference as compared to the spring and local water, results show that the addition of water to HFF-1 increased adhesion by 20%, but significantly impacted proliferation by 1-fold. Because alkaline water showed increased muscle hydration in studies discussed earlier, we wanted to see if it showed any differences in muscle cells.
L6
In this study we looked at the effects of various types of water, alkaline, spring, and local water, on cellular adhesion and proliferation of L6 cells on wells coated with 10µg/mL collagen. We will also be observing the cell morphology for any differences between each condition. Initial adhesion of L6 cells in various water types, alkaline water, spring water, and local water, on day 1 was analyzed. Figure 3A shows the greatest initial adhesion was observed in alkaline water at as compared to 100% media. Spring water and local water were observed to have similar percent adhesion. This indicates that HFF-1 cells adhered to the wells successfully. Cell proliferation was expressed in a percentage as compared to day 1 at 100% as seen in Figure 3B. The greatest cell proliferation was observed in local water at 560%, followed by alkaline water at 394%, and lastly, in spring water at 377%. Proliferation in all water types was about 1-2-fold less than media proliferation rates overall, while proliferation between water types was not affected. Proliferation of HFF-1 cells in local water on day 3 increased by less than 1-fold, while day 7 as compared to day 3 increased by 3-fold. Alkaline water and spring water showed similar proliferation rates, with about a 1-fold increase from day 1 to day 3 and remaining steady by day 7. Morphology of HFF-1 cells on day 1 was present and well rounded as shown in Figure 3C. On day 3, HFF-1 cells in water showed slightly difference morphology, especially in width. Alkaline water showed small, thin, elongated cells, local water showed mid-length cells with slightly thicker width, and spring water showed thickest width in cells with similar elongation. By day 7, cell density increased in alkaline water and media significantly while cells were observed to elongate slightly more as compared to day 3. While alkaline water did not show any significant difference as compared to the spring and local water, results show that the addition of water to L6 cells decreased adhesion, but did not affect cell proliferation as seen in HFF-1. Because it was observed that muscle cells appeared to be more tolerant of water, we wanted to further study its viability with the addition of ibuprofen.
The effect of various concentrations of ibuprofen on L6 cells
In this study we looked at the effects of low concentrations of ibuprofen on cellular adhesion and proliferation of L6 cells on wells coated with 10µg/mL collagen. We will also be observing the cell morphology for any differences between each condition. In our previous experiments, L6 cells were studied in high concentrations of ibuprofen greater than .05mg/mL. The data indicated that the concentrations were too high resulting in significantly low adhesion with observations of cell apoptosis by day 7. Initially we hypothesized that ethanol used to ensure solubility of the ibuprofen gel may have contributed to the decrease adhesion and cell apoptosis. We tested this theory by excluding ibuprofen to the stock solution and only adding ethanol. Analysis of the ethanol condition showing favorable results similar to that of cells in the control determining that ethanol did not play role in cell apoptosis. We focused on L6 cells in lower concentrations to continue to observe changes in cell behavior. Percent adhesion and proliferation was quantified and graphed in Figure 4A&4B. As shown in Figure 4A the highest percent adhesion on day 1 was observed in both 0.1mg and 0.05mg at 106% and 105% respectively when compared to the media control. The ethanol condition resulted in lowest percent adhesion of cells at 82%. Results from cell proliferation on days 3 and 7, as shown in Figure 4B, indicate that the ethanol environment provided highest percent proliferation overall compared to other conditions. The lowest concentration of ibuprofen at 0.05mg gave second highest percent proliferation on day 3 and by day 7 resulting in 1075% proliferation compared to day 1. Cells in all conditions experienced about a 2-fold increase on day 7 in comparison to day 3.
No significant change was observed in proliferation of all conditions compared to day 7 of media. Fluorescent images in Figure 4C show morphology of L6 muscle cells with initial circular shape on day 1. By day 3, fluorescent images show visibly fewer cells in conditions containing 0.01mg of ibuprofen. All conditions exhibited cell spreading and elongation by day 7.
Cell distribution in 3D fibrin beads and 3D printing
The purpose of studying various 3D environments is to establish the methodology of fibrin beads and 3D printing, and analyzing migration of L6 cells in these environments. In Figures 5A&5B, fluorescent images of L6 muscle cells in fibrin beads that were not placed on a matrix showed that muscle cells remained within the beads even up to day 7. Since no matrix was present in the wells, the cells did not spread to other locations from outside of the fibrin bead. This is most likely due to the lack of nutrients from the exterior surroundings. The cells visually depicted a behavior that indicates spreading and dispersing outward when present in matrices containing fibrinogen and thrombin. Two concentrations of the matrices were created using 20mg/ml and 10mg/ml of fibrinogen. Cells seemed to depict more spreading on the matrix created with 10mg/mL of fibrinogen than the 20mg/mL of fibrinogen. When observing 3D images taken of the cells that were plated using the 3D printer, we saw that cells did not remain uniform as compared to wells in fibrin beads. This may have been contributed to a nonhomogeneous mixture of the cells when combined with alginate/gelatin ink.

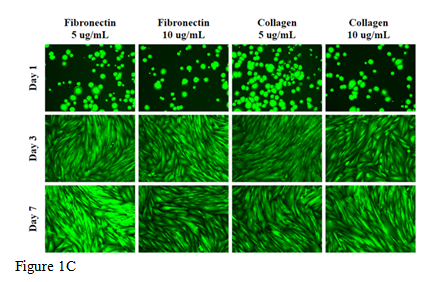
Figure 1 The effect of collagen and fibronectin on HFF-1 cells.
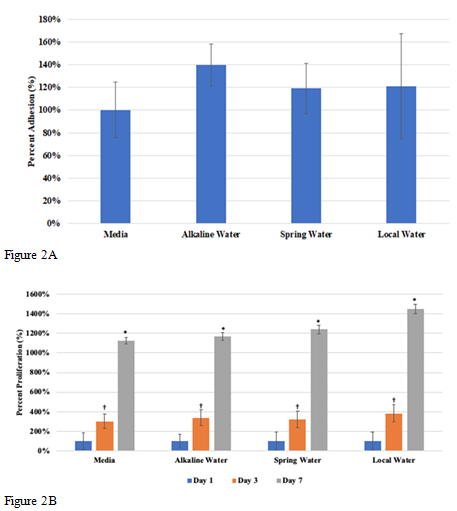
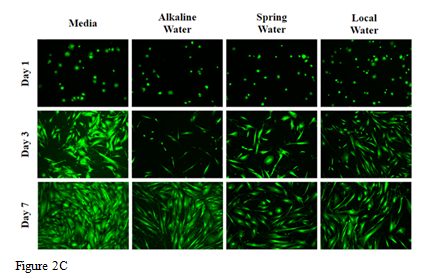
Figure 2 The effect of various water types on HFF-1 cells.
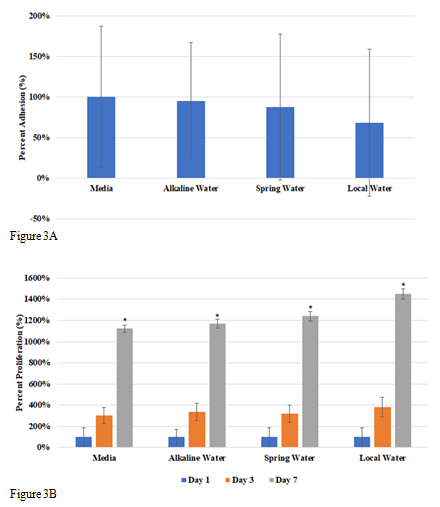
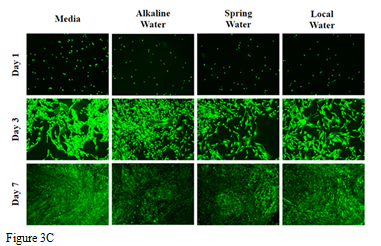
Figure 3 The effect of various water types on L6 cells.
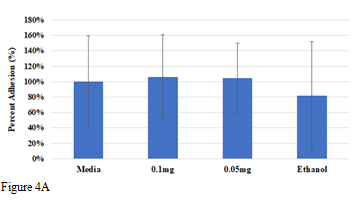

Figure 4 The effect of various concentrations of ibuprofen on L6 cells.
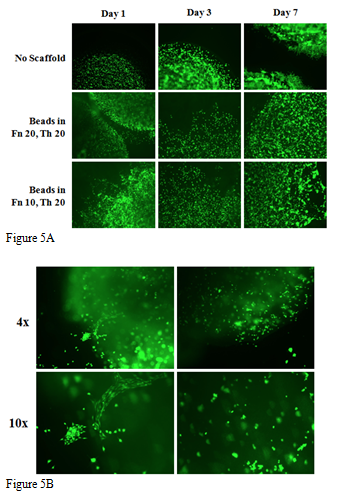
Figure 5 L6 Cell distributions in 3D fibrin beads and 3D printing.
An increasingly popular health trend that suggests that an alkaline diet will provide numerous benefits to the human body.1 Here, we observed the effects of alkaline water on muscle and nonmuscle cells. Fibronectin and collagen are extracellular matrix (ECM) proteins that promote cell adhesion and grown on trypsin treated cells.5 Collagen is a protein that is found abundantly in humans as it maintains the integrity of many connective tissues and ECM proteins.8 Fibronectin is a glycoprotein found in the ECM that binds to integrins after undergoing a configuration change.9 HFF-1 cells adhered highest in fibronectin coated wells (Figure 1A), while proliferation rates were seen to be highest in collagen coated wells and lower in fibronectin (Figure 1B). Initial adhesion after 1 hour was seen higher in fibronectin because cells require fibronectin to adhere to collagen and thus, not enough fibronectin was produced to allow for higher adhesion within the hour.3 Because cells adhered better to the fibronectin, it was likely more difficult for them to proliferate as contrasting results were observed between adhesion and proliferation. Future experiments in this study were performed using collagen coated wells because higher cell proliferation was observed as compared to fibronectin. A comparison of adhesion, proliferation, and morphology of HFF-1 and L6 cells with the addition of various types of water was studied. We determined that the water type (alkaline, spring, and local water) had no significant effect on cell adhesion, proliferation, or cell distribution within each cell line (Figure 2&3). However, it was observed that the addition of water had a significant impact on cell proliferation of HFF-1 cells but not L6 cells. According to the chemical composition breakdown of the human body, the skin is composed of 58% water while the muscle is composed of 70% water.10 Because muscles are made up of a larger water percent, addition of water to the L6 cells may not have been impacted proliferation as it did with the HFF-1 cell proliferation.
In previous experiments, higher concentrations of ibuprofen resulted in decreased cellular adhesion and proliferation of L6 muscle cells. Low concentrations at .1mg and .05mg did not show any significant impact in adhesion nor proliferation when compared to the control. This indicates that high doses of ibuprofen or anti-inflammatory drugs may be toxic and detrimental to muscle growth and strength. We observed very skewed data in highest concentrations of ibuprofen between 10mg to .25mg of ibuprofen. A previous study conducted by the Department of Physical Medicine and Rehabilitation in collaboration with Chang Gung Memorial Hospital and University in Taiwan indicated that the use of ibuprofen was directly related to the inhibition of Rb protein phosphorylation which is a protein that plays a critical role in the eukaryotic cell cycle for cell proliferation.5 Our data from high concentrations of ibuprofen may help to support this theory however, the lowest concentration at 0.05mg showed a slight increase in proliferation compared to the controls, but was not significant. This could possibly mean that lower concentrations of ibuprofen may aid in increased cellular proliferation, or that the ibuprofen concentration was too low and did not provide a meaningful difference in cell behavior. Other studies examining the effects of ibuprofen on cell migration of coronary artery smooth muscle cells support our data where high concentrations of ibuprofen resulted in significant inhibition of cell migration, but lower concentrations did not affect cell migration.11 When observing 3D fibrin matrices using thrombin and fibrinogen, there was a distinct change in proliferation of the L6 muscle cells in fibrin beads when seeded on these matrices. Cells exposed to the highest concentration of fibrinogen displayed a decrease in cell migration. Both matrices contained the same concentration of thrombin. Previous research indicates that thrombin actively cleaves certain amino acid regions which results in new binding regions to be exposed.12 The exposure of these new regions helps to activate receptors that are known to be present on fibroblast cells.12 The specific activity would help to promote synthesis of existing fibroblast cells allowing the cells to spread and proliferate.12 Although L6 muscle cells were used in our study, we can assume that due to the corresponding findings from our results, L6 muscle cells also contain a similar receptor activated by the enzymatic activity from thrombin. Fibrinogen on the other hand, provided us with contrasting results where higher concentrations of the protein may have caused a decrease in cell proliferation. When fibrinogen and thrombin are combined, fibrinogen is converted into fibrin strands.13 Fibrin is considered a polymer that serves as a scaffold for cellular migration.14 It may be that a higher concentration of fibrinogen would result in an increase of the fibrin gel density.14 Therefore, a more dense material containing a tight network of fibrin strands may not allow for an effective pore size for cells to proliferate.14 If this holds true, it would be important to consider fibrinogen levels when creating a favorable 3D environment for cells. As previously mentioned in our results of L6 cells in 3D printed ink, cells did not remain uniform as compared to wells in fibrin beads. This may have been contributed to a nonhomogeneous mixture of the cells when combined with alginate/gelatin ink. The composition of the ink and ink viscosity is crucial in developing an effective scaffold with 3D printing.15 Alginate and gelatin are commonly used materials for ink composition however, the addition of other biomaterials like collagen and ECM would improve the cell-activating properties and development of 3D construct when using extrusion-based 3D printing.14 Furthermore, cell densities play an impact on viscosity of the extrusion ink.16 Increased cell densities of the material can decrease viscosity substantially and affect the performance of extrusion-based 3D printing.16 For future studies, we will explore 3D environments of L6 cells with various water types and ibuprofen concentrations. We will also explore the levels of fibrinogen used to create 3D matrices, and evaluate cell density and biomaterial composition in 3D printing.17
This study was performed to determine the morphology, initial adhesion, and proliferation behavior of L6 muscle cells when exposed to the different environments that are expected to impact muscles at the tissue level. It was concluded that alkaline water did not show significant differences in behavior between the varying water types, and the addition of water itself did not affect muscle cell proliferation. Higher concentrations of ibuprofen resulted in a significant decrease in cellular adhesion and cellular apoptosis by the end of previous experiments. When studying low concentrations of ibuprofen, concentrations less than 0.05mg/mL had no effect on L6 cell behavior. During observation of L6 cells in 3D environments, cells migrated the best in fibrin beads on matrices with lower concentrations of fibrinogen (10mg/mL). To further understand the impact of water and ibuprofen on L6 cells additional proliferation studies will need to be conducted in optimal 3D environments.
The authors would like to thank and Melissa McCoy for supporting the group through the experiments and encouraging the research.
Authors declare that there is no conflict of interest.

©2019 Jennifer, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.