Advances in
eISSN: 2572-8490


Research Article Volume 9 Issue 1
Correspondence: Deryabina OG, Institute of Genetic and Regenerative Medicine, M. D. Strazhesko National Scientific Center of Cardiology, Clinical and Regenerative Medicine, National Academy of Medical Sciences of Ukraine, Ukraine
Received: August 03, 2023 | Published: September 18, 2023
Citation: Konovalov SV, Moroz VM, Husakova IV, et al. Comparative influence of mesenchymal stromal cells of different origin on DNA fragmentation of neuronal nuclei during ischemia-reperfusion of the somatosensory cortex of the rat brain. Adv Tissue Eng Regen Med Open Access. 2023;9(1):29-33. DOI: 10.15406/atroa.2023.09.00138
Relevance: One of the main causes of stroke in acute cerebrovascular accident (ACVA) is ischemia, which begins with the formation of an acute neuronal energy deficit with subsequent activation of the "ischemic cascade" reactions that lead to irreversible damage to nervous tissue.
Aim: To compare the effect of mesenchymal stromal cells (MSCs) of different origin and human MSCs from Wharton's jelly lysate on neuroapoptotic changes in the somatosensory cortex of the rat brain in conditions of model ischemia-reperfusion (IR) performed by ductal cytoflowmetry.
Materials and methods: The experiment was carried out using 165 four-month-old male Wistar rats weighing 160-190 g, which were subjected to bilateral 20-minute transient ischemia-reperfusion (IR) of the internal carotid arteries. After modeling the pathology, the animals were injected into the femoral vein (iv) with MSCs obtained from umbilical cord Wharton jelly, human and rat adipose tissue in the amount of 106 cells/animal. Other groups of experimental animals were intravenously injected with fetal rat fibroblasts in the amount of 106 cells/animal (in 0.2 ml of physiological solution) and MSCs from umbilical cord Wharton's jelly lysate in a dose of 0.2 ml/animal. Control animals were injected intravenously with 0.2 ml of physiological solution. The level of DNA fragmentation in the nuclei of neurons of the somatosensory cortex of rats on the 7th day after ischemia-reperfusion was studied by flow cytometry. The research was carried out on a flow cytometer "Partech РАС" of the company Partech, Germany. The statistical significance of the differences was assessed by Student's t-test.
Results: The study noted an increase in the level of fragmented DNA in a group of animals with IR by 3.25 times 7 days after model IR. The performed treatment showed that in groups with transplanted MSCs of various origins and MSC lysate from human Wharton's jelly cells, the intensity of DNA fragmentation in the nuclei of neurons in rat brain somatosensory cortex reliably decreased in1.8-2. 6 times compared with the group of control pathology (IR without treatment).
Conclusions: Experimental 20-minute IR of the brain of rats forms a persistent focus of necrotic and apoptotic death of neurons, which is manifested by an increase in fragmented DNA (3.25 times). Intravenous transplantation of MSCs of various origin and lysate of MSCs from human Wharton jelly has a therapeutic effect in model IR, which is manifested by a decrease in the processes of neuro-destruction and neuroapoptosis in the area of ischemic brain damage Such effect is a link to the polytrophic mechanism of MSCs neuro-protective action.
Keywords: ischemia-reperfusion, somatosensory cortex, flow cytometer, mesenchymal stromal cells, rats
MB, Mitochondrial diseases; mtDNA, mitochondrial DNA; MRI, magnetic resonance imaging; GTCS, generalized tonic-clonic seizure; HSV, herpes simplex virus; ENMG, electroneuromyography
One of the main causes of stroke in acute cerebrovascular accident (ACVA) is ischemia, which begins with the formation of an acute neuronal energy deficit with subsequent activation of the "ischemic cascade" reactions, which leads to irreversible damage to nervous tissue.1 To develop new methods of pathogenetic therapy of ischemic stroke, it is advisable to find out which type of cell death prevails during the development of ACVA. From the standpoint of evidence-based medicine, thrombolytic therapy is currently the main therapeutic measure for ischemic stroke.2–4 However, restoration of perfusion of ischemic tissue contributes to the deepening of violation of metabolic processes in the brain, which leads to the formation of reperfusion injuries.1,5–7 The destruction of desmosomes and an increase in the distance between individual neurons promotes the free spread of free radicals and secondary messengers, which leads to the damage of intact cells and neurons’secondary damage. In conditions of ischemia-reperfusion (IR, clinically this model corresponds to the post-perfusion injury of the brain after thrombolysis), the prevailing number of neurons will die by apoptosis.8 Therefore, the main direction of cyto-protection can be considered as the development and subsequent clinical application of new approaches that are able to prevent and inhibit the very phenomenon of apoptosis. Taking this into account, it would be appropriate to characterize and compare the effect of mesenchymal stromal cells (MSCs) of different origin and MSCs lysate from human Wharton's jelly on neuroapoptotic changes in the somatosensory cortex of rat brain in the condition of model IR to assess neuronal DNA fragmentation. This can be performed by the method of ductal flow cytometry, which is generally recognized and one of the best methods.
Animals. For the experiment, 165 sexually mature white male rats of the Wistar line, four months old, with an initial body weight of 160-190 g were used. Animals from the vivarium of the Vinnytsia National Medical University named after M.I. Pirogov were kept in standard conditions with free access to water and food. When conducting the research, the methodological recommendations of the Ministry of Health of Ukraine and the requirements of bioethics regarding the National "General Ethical Principles of Animal Experiments" approved by the 1st National Congress on Bioethics (Kyiv, Ukraine, 2001) and the Law of Ukraine "On the Protection of Animals from Cruelty Treatment" dated February 26, 2006 were strictly followed.
Ischemia-reperfusion model. The experimental model of IR was developed by placing ligatures on the internal carotid arteries on both sides under propofol anesthesia (Propofol-novo, Novofarm-Biosintez LLC, Ukraine, 60mg/kg) lasting 20 minutes. The selected model reflects the clinical picture of brain infarction and is adequate for the experimental study of potential neuroprotective substances.9 MSCs of various origin in the amount of 106 cells/animal in 0.2 ml of 0.9% NaCl solution were injected once intravenously into the catheterized femoral vein immediately after IR. The studied substances were injected into the femoral vein once immediately after IR, because according to literature data earlier transplantation of MSCs led to more significant neurological recovery and reduced infarct volume, and also required a smaller number (1×106) of donor cells for a beneficial effect.10–13 The obtained results are a continuation of our earlier studies.14
Experimental animals were divided into 7 groups (Table 1).
|
Animal group |
Animal number |
Treatment |
|
1st group |
10 |
pseudo-operated animals + 0.9% NaCl solution at a dose of 2ml/kg |
|
2nd group |
40 |
IR+0.9% NaCl solution at a dose of 2 ml/kg |
|
3rd group |
20 |
IR+MSC from human Wharton’s jelly at a dose of 106cells/animal |
|
4th group |
20 |
IR+rat fetal fibroblasts at a dose of 106cells/animal |
|
5th group |
25 |
IR+MSC from human adipose tissue at a dose of 106cells/animal |
|
6th group |
25 |
IR+stem cells from rat adipose tissue at a dose of 106cells/animal |
|
7th group |
25 |
IR+MSCs lysate from Wharton's jelly in a dose of 0.2ml/animal |
Table 1 Distribution of animals in the experiment
The 1st group included sham-operated rats, which were sequentially subjected to the following interventions (anesthesia, skin incision, vascular preparation (except for ligation of the internal carotid artery) (ICA)), which reproduced the effect of the traumatic conditions of the experiment. The 2nd group is a group with control pathology. Rats of this group were subjected to 20-minute ischemia of the brain (I) by applying ligatures to the ICA. After 20 minutes, the ligatures from the ICA were removed (reperfusion) (R) and 0.9% NaCl solution at the dose of 2 ml/kg was injected once into the femoral vein. A similar dose was administered to rats of the 1st group. The 3rd group of animals immediately after IR was transplanted with MSCs from human Wharton’s jelly in the amount of 106 cells/animal. The 4th group of animals underwent a single transplantation of fetal rat fibroblasts at a dose of 106 cells/animal immediately after IR. The 5th group of animals with IR received MSCs of human adipose tissue at a dose of 106 cells/animal. The 6th group of animals was injected immediately after IR with stem cells obtained from rat adipose tissue in the amount of 106 cells/animal. Immediately after IR, the 7th group of animals was given a single injection of MSC lysate from human Wharton cells in a volume of 0.2ml/animal.
In order to analyze the influence of MSCs of different origin and lysate of MSCs from human Wharton’s jelly on the dynamics of neuroapoptotic changes in the somatosensory cortex of the rat brain 7 days after IR (subacute period of ischemia), the brains of rats were rapidly removed immediately after decapitation with anesthesia by pentobarbital ("Penbital", Bioveta JSC, Czech Republic, 100 mg/kg).15–17
It is known that the process of neuroapoptosis begins with the destruction of the cell nucleus and damage to deoxyribonucleic acid (DNA), so the presence of fragmented DNA is a marker of apoptosis.18 The level of DNA fragmentation in the nuclei of neurons of the somatosensory cortex of the rat brain on the 7th day after IR was studied by flow cytometry.19 To obtain nuclei suspension, SuStain DNA from Partec (Germany) was added to the brain tissue according to the manufacturer's instructions. CellTrics 50 μm disposable filters (Partec, Germany) were used. Nuclei suspensions of sensorimotor cortex biopsies were prepared immediately after the material was collected and washed with a cold (+4±80C) phosphate buffer with pH 7.4 (Sigma). The research was carried out on a flow cytometer "Partech РАС" (Partech, Germany). An ultraviolet lamp was used to excite the fluorescence of the nuclei DNA label- diamidinophenylindole. 20,000 events were analyzed from each sample of nuclei suspension. Flow analysis of DNA fragmentation was performed using FloMax software (Partech, Germany) by highlighting Sub-G1 regions on DNA histograms. The statistical significance of the differences was assessed by Student's t-test.
The study performed showed statistically significant increase by 3.25 times in the level of fragmented DNA in the group of animals with IR (group 2) 7 days after modelling IR in rats (Figure 1A&1B). Such dynamics indicate the formation of brain tissue lesions through neuroapoptosis.

Figure 1A RN1 (Sub-G1) – 4.18%. DNA fragmentation in the nuclei of neurons of somatosensory cortex of pseudo-operated rats (group 1).
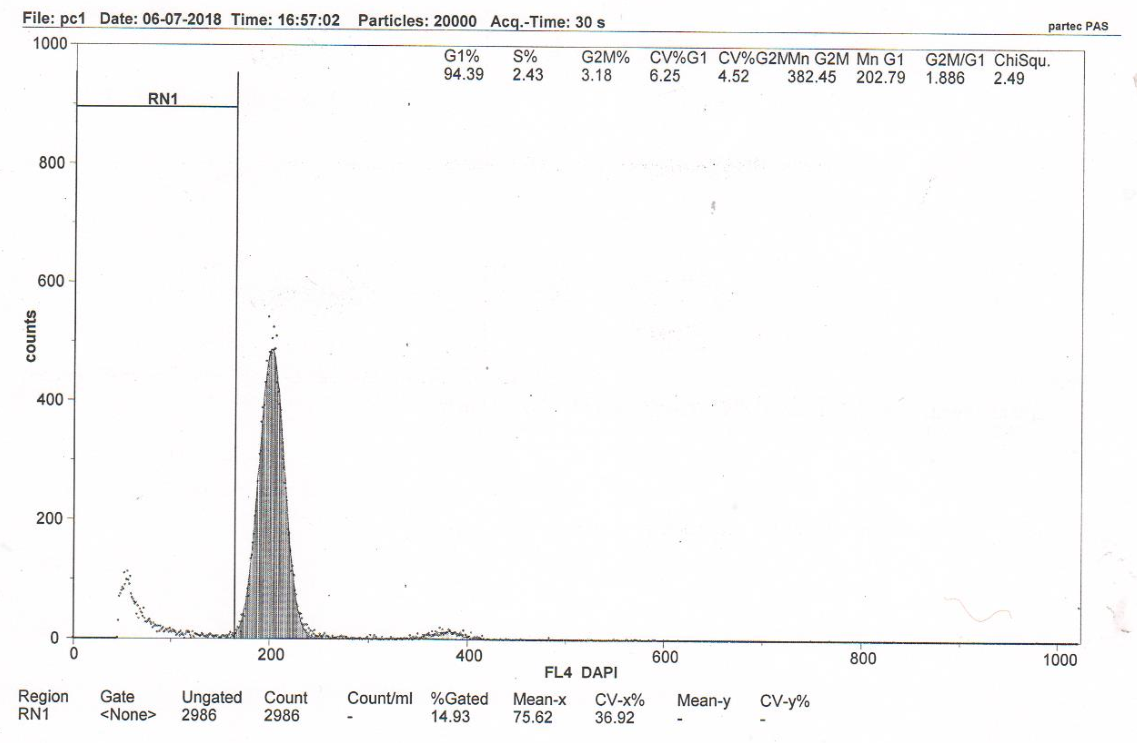
Figure 1B B RN1 (Sub-G1) – 13.58%. DNA fragmentation in the nuclei of neurons of somatosensory cortex of rat brains from the control pathology group (group 2) (cerebral IR). Flow cytometry.
The treatment carried out showed that in groups with transplanted MSCs of various origin (groups 3-6) and lysate of MSCs from human Wharton’s jelly (group 7), the intensity of DNA fragmentation in the nuclei of neurons in somatosensory cortex 7 days after the modelling of the cerebral 20-minute IR reliably decreased in 1.8-2.6 times compared with the group of control pathology (group 2, IR without treatment) (Figures 2&3A-E).

Figure 2 The effect of mesenchymal stromal cells of different origin and MSCs lysate on nuclei DNA fragmentation of neurons of the somatosensory cortex of rats with cerebral reperfusion after 7 days (M±SD, n=5).
*–statistically significant difference compared to sham-operated animals
(р<0.05); #–statistically significant difference compared to ischemic animals
(p<0.05).
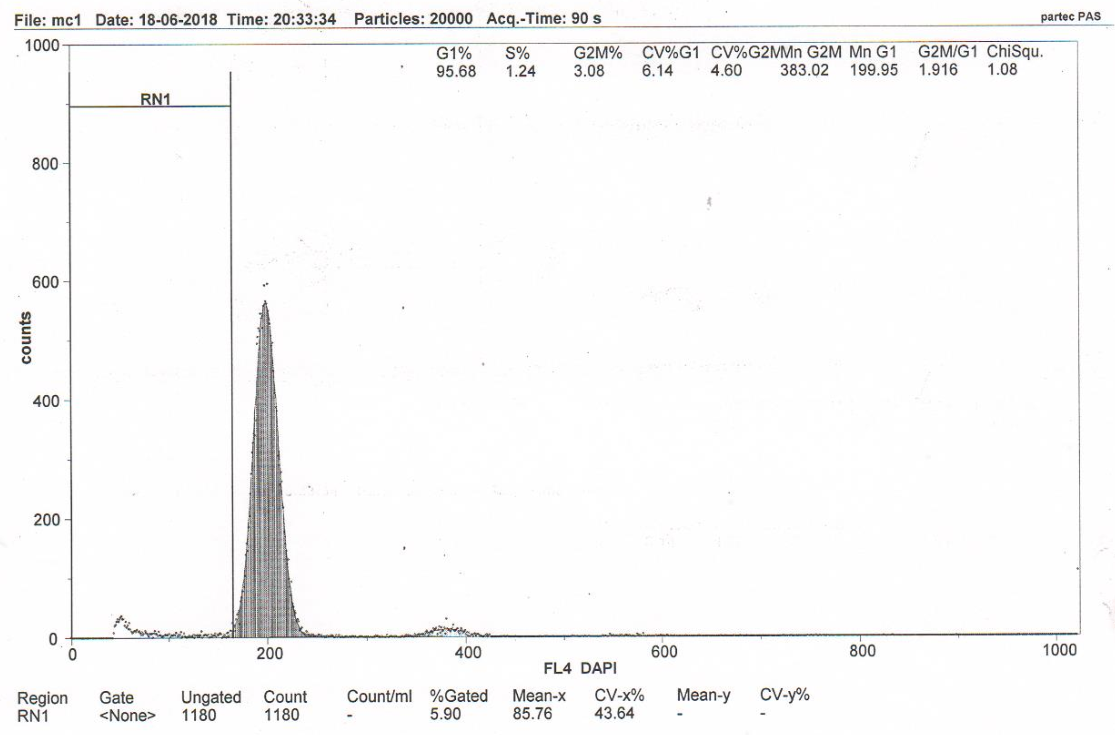
Figure 3A RN1 (Sub-G1)–5.57%. DNA fragmentation in the nuclei of neurons of the somatosensory cortex of the rats with model cerebral IR with transplantation of MSCs from human Wharton’s jellyb (group 3).
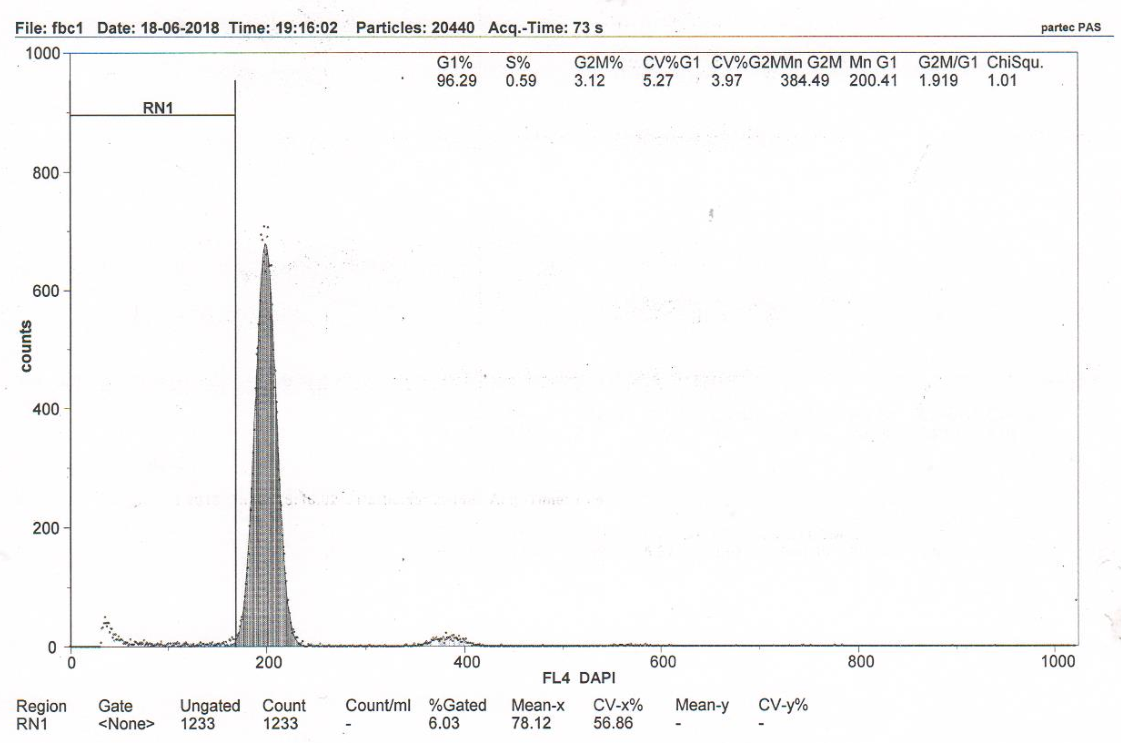
Figure 3B RN1 (Sub-G1)–5.96%. DNA fragmentation in the nuclei of neurons of the somatosensory cortex of the rats with model cerebral IR with transplantation of rat fetal fibroblasts (group 4).
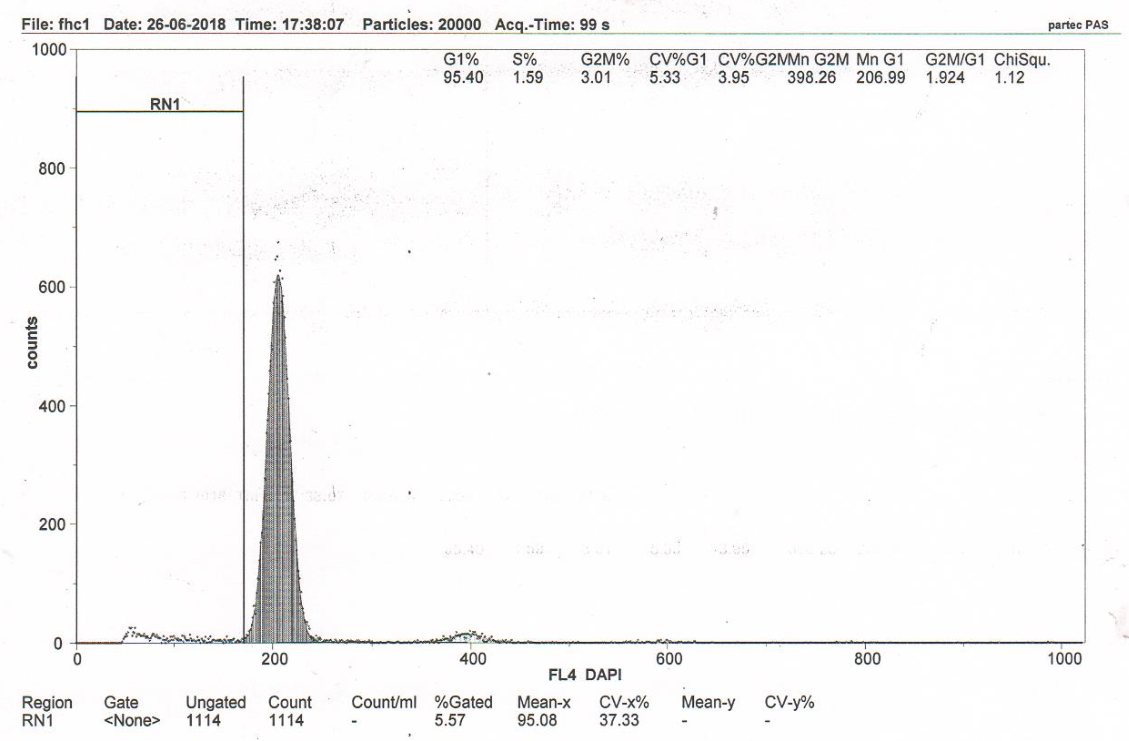
Figure 3C RN1 (Sub-G1)–5.19%. DNA fragmentation in the nuclei of neurons of the somatosensory cortex of the rats with model cerebral IR with transplantation of MSCs from human adipose tissue (group 5).
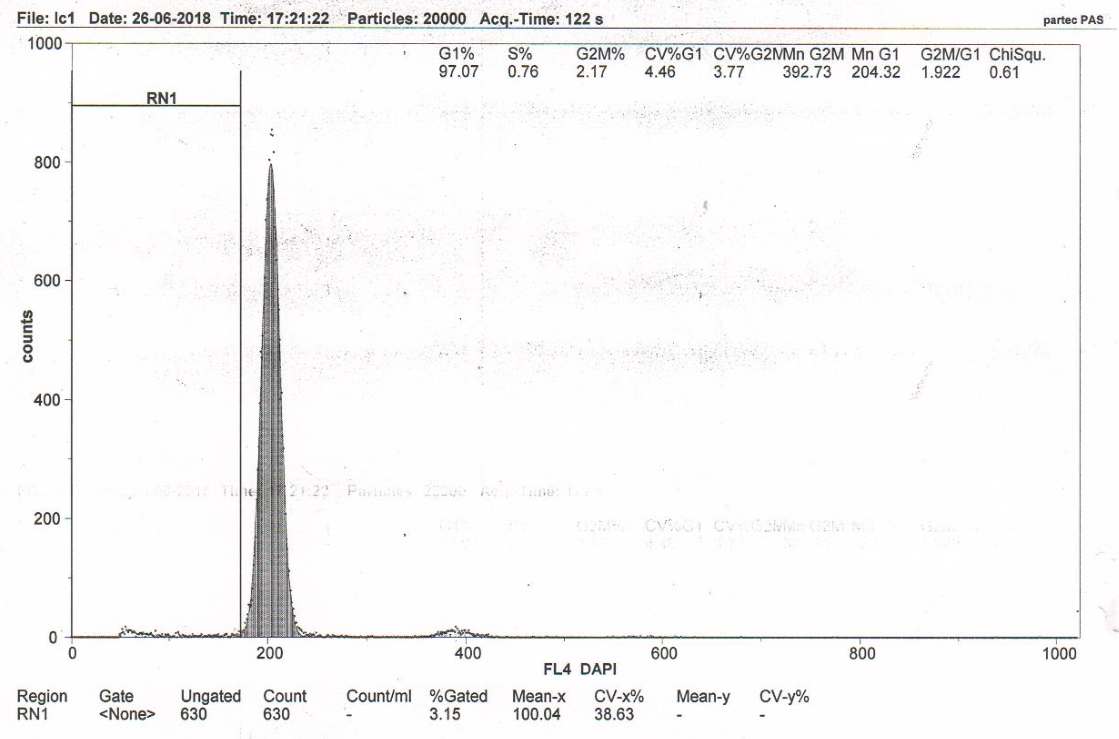
Figure 3D RN1 (Sub-G1)–7.68%. DNA fragmentation in the nuclei of neurons of the somatosensory cortex of the rats with model cerebral IR with transplantation of MSCs from rat adipose tissue (group 6).
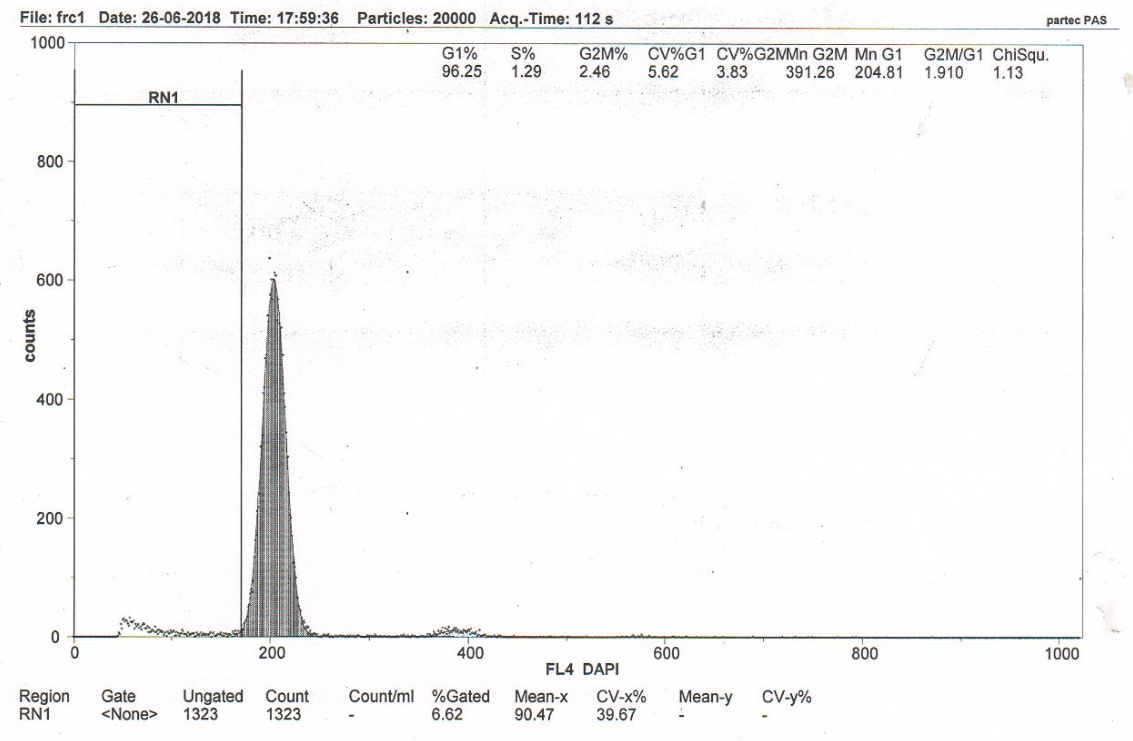
Figure 3E RN1 (Sub-G1)–7.18%. DNA fragmentation in the nuclei of neurons of the somatosensory cortex of the rats with model cerebral IR with intravenous administration of MSC lysate from human Wharton’s cells (group 7). Flow cytometry.
Transplantation of MSCs obtained from Wharton's jelly and human adipose tissue, as well as fetal fibroblasts and MSCs from rat adipose tissue and MSC lysate from human Wharton's jelly, in rats with IR had a cerebroprotective effect similar in orientation and strength. This is indicated by a reliable decrease (compared with the control, group 2) of DNA fragmentation in the nuclei of the somatosensory cortex neurons during the studied period by an average of 58.98%, 56.11%, 61.78%, 43.45% and 47.13%, respectively (Figure 2).
Thus, inhibition of the intensity of neuroapoptosis in the somatosensory cortex of the rat brain under the influence of MSCs of various origins and MSCs lysate from human Wharton's jelly indicates a reduction in the focus of the ischemic penumbra due to the preservation of morphologically intact neurocytes number and is one of the mechanisms of cerebroprotective action in post-reperfusion brain damage.
Our study demonstrates the therapeutic effects of intravenous transplantation of MSCs of various origin and MSCs lysate from human Wharton’s jelly after IR modelling, which was created by applying ligatures to the internal carotid arteries on both sides lasting 20 minutes. Intravenous administration of MSCs of various origin and MSCs lysate after 20-minute IR led to the decrease in the level of fragmented DNA, a decrease in apoptosis, and an increase in intact neurons in the somatosensory cortex 7 days after IR in rats. The results of our study echo the recently obtained results, which show that treatment with MSCs reduces the extent of damage and inflammation, improves neuroprotection after IR brain injury in rats.20–22 Thus, intravenous transplantation of MSCs can increase the therapeutic efficiency of reperfusion therapy in cerebral ischemia, which indicates the prospect of their use in cell therapy of acute cerebral ischemia.
None.
The authors declare that there are no conflicts of interest.
None.

©2023 Konovalov, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.