Advances in
eISSN: 2572-8490


Research Article Volume 5 Issue 2
Institute of Science and Technology, Federal University of Alfenas, Brazil
Correspondence: Maria do Socorro Fernandes Melo, Institute of Science and Technology, Federal University of Alfenas, Poços deCaldas-MG, Brazil,
Received: August 06, 2019 | Published: August 15, 2019
Citation: Melo MDSF, Almeida LAD, Castro KCD, et al. Chitosan membrane incorporated with Passiflora edulissims extract for potential application as wound dressing. Adv Tissue Eng Regen Med Open Access. 2019;5(2):103-108. DOI: 10.15406/atroa.2019.05.00107
The aim of this work was to extract, characterize and incorporate the extract of Passiflora edulis in to chitosan membranes for potential application as wound dressing. The extract was obtained from leaves using hydroalcoholic maceration. The extract (EPE) was analyzed by LC-Mass and Infrared Spectroscopy for identification of characteristic compounds, and further incorporated to chitosan membrane (QEPE). The membranes were obtained by solvent evaporation technique and characterized by morphology (SEM), swelling behavior, moisture content, water-solubility, water vapor permeability, and thermal stability. The interactions between chitosan and the extract were also analyzed by infrared spectroscopy. The presence of flavonoids (hydrophilic compounds) in EPE affected the moisture content, water-solubility and water-vapor permeability of QEPE by increasing these properties in respect to the values found for the chitosan only membrane (QF). No significant difference was observed in the swelling ratio of both membranes. Moreover, a reduction in the enthalpy of fusion and hence in the degree of crystallinity was observed for QEPE, probably due to the presence of phenolic compounds in the extract that difficult orientation and organization of the polymeric chains due to the steric effect. In conclusion, QEPE was flexible, transparent, and homogeneous; demonstrated fluid absorption capacity, water vapor permeability and thermal stability under physiological conditions. Therefore, it has great potential for application in the treatment of skin wounds.
Keywords: Passiflora edulis, chitosan, wound healing, membrane, biomedical applications
The concern about wound care is ancient, which has driven the technological advance. In recent years, a number of topical wound care products have been found on the market, such as ointments, emulsions, gels, creams and solutions. Occlusive bandages are also available and are able to promote wound healing in a wet environment and keep the wound tissue hydrated. Nevertheless, they lack on drugs that can help in reducing pain as well as in the inflammatory or healing processes. Film-forming materials represent a convenient alternative to conventional bandage systems and they have the advantage of making the treatment more comfortable and functional. In addition, the ability to controlled or prolonged release of the drug used in treatment decreases the number of times needed to change the curative, which decreases the dose administered and avoids pain and damage to the healing process during curative removal, providing increased patient compliance with treatment.1
Several polymers have been studied for biomedical applications, such as controlled release systems. In matrix systems the drug may be homogeneously dispersed in the polymer matrix, adsorbed on its surface or within a reservoir. Drug release involves physical and chemical processes such as: penetration of water into the matrix, diffusion of the drug through the pores of the matrix, polymer degradation or a combination of the latter two mechanisms.2 Chitosan, as it has unique properties, is recommended as a suitable functional material due to its excellent biocompatibility, biodegradability, and low toxicity and adsorption properties. Interesting biological properties of chitosan also include bactericidal, hemostatic and antitumor activities, as well as promoting wound healing.3 In addition, chitosan gradually de-polymerizes by releasing N-acetyl-BD glucosamine, which initiates fibroblast proliferation and also aids in collagen deposition and stimulates increased level of natural hyaluronic acid synthesis at second wound sites.4 Due to its important characteristics, chitosan has been widely used in the preparation of cell growth supports and matrices for controlled drug release. In addition to conventional drugs, herbal medicines and extracts can also be immobilized and released gradually from these matrices, which are designed according to the release rate indicated for each type of drug/treatment.5
In the middle of the last century, with the rise of a therapy centered on the use of synthetic drugs, the use of herbal medicines declined. However, in recent decades, the use of natural products has been reborn due to the bacterial resistance to traditional antibiotics and other side effects caused by synthetic drugs. The use of medicinal plants has been increased in both developed and in development countries.6 For instance, in Brazil, a policy to encouraging the use of herbal medicine, among other therapies, was presented by the federal government.7,8 This public policy aimed to ensure the safe and correct access of medicinal and phyto-therapeutic plants by the population, as well as to stimulate production of these products.6 One of the medicinal species listed by the Brazilian Ministry of Health with great potential for use, is Passiflora. Passiflora spp leaves are used as anti-inflammatory, have antimicrobial activity and are known for their action in cases of insomnia and irritability. These properties are attributed to the species Passiflora incarnata L. and Passiflora alata Curtis, Passifloraceae,.9 In addition, previous studies reported that the use of Passiflora edulisleaf extract in wound healing has led to increased fibroblast proliferation and improved epithelization.10 Gonçalves & Collaborators11 studied the effects of hydroalcoholic extract of Passiflora edulis leaves on urinary bladder healing and related to an increase in fibroblast proliferation and collagen formation.11 Passiflora edulis extract is composed by flavonoids, such as vitexin, isovitexin, orientin, isorientin, apigenin, and kaempferol. These molecules have interesting properties, such as antioxidant and anti-inflammatory activities; and are able to inhibit aggregation of blood platelets.11,12
In this context, this work aimed to extract, characterize and incorporate extract of Passiflora edulis in chitosan-based membranes for potential wound healing application. The extract was obtained by maceration, analyzed by Mass and Infrared Spectroscopy, and further incorporated to chitosan membranes. The membranes were obtained by solvent evaporation technique and characterized by morphology, swelling behavior, humidity, solubility, water vapor permeability, and thermal stability. The interactions between chitosan and the extract were analyzed by infrared spectroscopy.
The leaves of Passiflora edulis were collected in September 2014, the municipality of Alfenas-MG having the following GPS coordinates latitude 21º 24 '54', 16''S, longitude 45º 14.78 '' W at 786.8 m. The samples were identified and the exsiccate deposited under registration No. 2542.
Preparation and characterization of the extract of P. edulis leaves
The dried and pulverized P. edulis leaves were submitted to exhaustive maceration in 70% (v/v) hydroethanolic solution. After this, the mixture was filtered and the organic solvent was removed using a rot vaporator and the water removed by lyophilization. The obtained raw leaf extract of Passiflora edulis (EPE) was stored in a freezer at -20ºC.
The extract was analyzed by Mass Spectrometry (FIA-ESI-IT-MSn) and infrared spectroscopy. Mass spectra were obtained on the Thermo Scientific® LCQ fleet spectrometer equipped with a direct sample insertion device via continuous flow injection analysis (FIA). The sample was electronized by electrospray (ESI) and fragmentation was obtained in multiple stages (MSn), in an ion-trap inter phase (IT). The negative mode was chosen for the generation and analysis of all spectra. The experimental conditions were: -35V voltage, -500V spray voltage, 350°C capillary temperature, carrier gas (N2) and flow 60 (arbitrary units). The acquisition range was m/z 100-2000, with two or more scanning events performed simultaneously in the spectrum. The infrared spectrum of the extract was collected in an Agilent Technologies Spectrophotometer model Cary 630 from 650 to 4000cm-1.
Preparation and characterization of the membranes
Medium molecular weight chitosan powder (Sigma-Aldrich, USA) with 75% to 85% deacetylation grade was used in the preparation of the membranes by solvent evaporation (casting). The chitosan only (QF) membrane and the membrane incorporated with the Passiflora edulis extract (QEPE) were prepared from a 2% chitosan solution using 0.1% acetic acid as solvent. 5% EPE (m/v) was added to the chitosan solution in order to prepare QFEPE. Then, 10mL of the solution was poured into 14cm diameter polypropylene Petri dishes, dried at room temperature until completely detachment of the membrane from the mold.
Thickness
For each sample, thickness was measured in ten different positions to obtain the average. A bench micrometer, Digimatic Micrometed was used to get the measurements of thickness.
Morphology
Morphology was evaluated by Scanning Electron Microscopy (SEM, JEOL JSM-7500F Field Emission Scanning Electron Microscope). Membranes were previously dried in a desiccator with silica for 24hours, and then coated with a 16nn thickness gold in a Sputter Coater (BalTec, model SCD050) for 60seconds at 2×10−2 Pa.
Moisture content
The moisture content was determined by the gravimetric method. The initial mass (Mi) of the membranes were obtained in a scale (SHIMADZU model AY220). They were then conditioned in an air circulating oven at 105°C for 24hours and weighted again (Mf). The moisture content (%moisture) was calculated from Equation 1:
Eq.1
Water solubility
The same membranes used in the moisture content determination were immersed in an Erlenmeyer with 50mL of distilled water and slowly stirred in Dubnoff shaker for 24hours at 25°C. After this period, membranes were removed and taken to the oven at 105°C for 24hours and weighted again (Ms). After this, the percentage of water-soluble material (%WS) was calculated by using Equation 2:
Eq.2
Swelling behavior
Initially, the films were placed in a desiccator for a period of 24hours and weighed thereafter (Wi). They were then immersed in approximately 10mL of distilled water and placed in an oven at 37°C (to simulate body temperature) for 24 hours and weighed again (Wf). The swelling ratio (%S) was then calculated by Equation 3:
Eq.3
Water vapor permeability
The coefficient of permeability of water vapor (WVP) was determined gravimetrically according to the ASTM E96-95. Membranes were cut in order to have a permeation area of 15.21cm2. The cell, containing anhydrous calcium chloride, were placed in a hermetically sealed container and maintained in 75% of relative humidity at 25°C. The cells were weighed after 2hours and then at periodic intervals of time, for 72hours. Water vapor permeability coefficient was calculated by Equation 4:
Eq. 4
where WVP is water-vapor permeability coefficient
G is mass gain per day (g/day),![]() is average thickness of membranes (mm), A is exposed area to mass transfer (m2), and ΔPw is partial pressure difference across the membrane (kPa).
is average thickness of membranes (mm), A is exposed area to mass transfer (m2), and ΔPw is partial pressure difference across the membrane (kPa).
Thermal analysis
Thermal analysis was performed on a Calorimeter DSC 200-F3 (NETZSCH) from 0°C a 300°C at a heating rate of 10°C/min, under nitrogen atmosphere. The melting temperature (Tm) and the enthalpy of fusion (ΔHm) of the membranes were determined and the crystallinity degree (Xc) was calculated by Equation 5:
Eq. 5
Infrared spectroscopy
The infrared spectra of the membranes were collected in an Agilent Technologies Spectrophotometer model Cary 630 from 650 to 4000cm-1.
LC-ESI-MS and FIA-ESI-IT-MSn results indicated the presence of flavonoids, especially C-heterosides (Table 1). Flavonoids and condensed tannins have anesthetic activity, can act as sterilizing due to their bactericidal and antiviral properties, in addition to promote cell regeneration that are important characteristics for wound healing. In terms of chemical structure, flavonoids have tricyclic compounds with radicals attached to their rings.13 After characterization, the EPE was incorporated to a chitosan membrane (QEPE) that would serve as a matrix for sustained release of the extract during the healing process. The thickness of QEPE was around double of QF one (Table 2). In despite of thickness, both QF and QEPE have similar characteristics such as flexibility, transparency and homogeneity. Figure 1 show the SEM micrographs for QF and QEPE where the homogeneous distribution of the extract along the polymeric matrix can be observed. This homogeneous distribution was also confirmed by the low standard deviation value found for the thickness measurements, indicating the compatibility of the extract with the chitosan matrix and its uniform distribution along in the membrane. The increase in QEPE thickness can be also associated to the moisture content of this membrane, which corresponds to twice the value found for QF (Table 2). This can be attributed to the hydrophilic characteristic of the extract, which contains phenolic acids and their derivatives. These compounds are natural hydrophilic antioxidants and receptive to moisture.14 As expected, an increase on QEPE solubility was also observed in respect to QF. However no significant difference was observed in the swelling behavior of both membranes (Table 1). These properties (moisture, swelling, solubility) are important due to the preservation of membrane integrity mainly in wet environments, such as in the healing process (PENG et al. 2013).15 Regarding to the water vapor permeability, QEPE membrane was more permeable to water vapor than QF (Table 2). The exchange of gases with the environment is conducive to having a proper treatment of wounds, since the skin performs this function. A dressing should at the same time keep the wound site moist and absorb excreted exudate from the wound to prevent fluid buildup between the wound and the dressing, and avoid microorganism contamination.
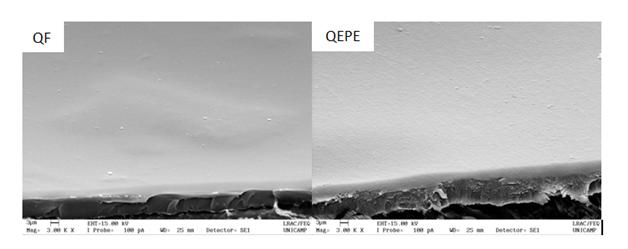
Figure 1 Micrographs of chitosan membrane (QF), and chitosan membrane incorporated with the extract of Passiflora edulis leaves (QEPE).
S. No |
Proposed compound |
MS (m/z) |
Fragments MS (m/z) |
Reference |
1 |
Lutein-7-0-pyranosyl-3-0-glycoside or Lutein-6-C-pyranosyl-8-C-Glycoside |
609 |
519; 489; 447; 462; 301 |
PIA et al., 2015 |
2 |
Apigenin-6-8-di-C-glycoside |
593 |
325; 297 |
Zuccolotto et al., 2011 |
3 |
Apigenina-6-C-arabinosideo-8-C-glicosideo |
563 |
504; 473; 443 |
Colombo et al., 2008 |
4 |
Apigenin-6-C-arabinoside-8-C-glycoside |
447 |
327; 357 |
PIA et al., 2015 |
5 |
Isovetexin |
431 |
341; 311 |
PIA et al., 2015 |
6 |
4-0-D-Glycopyranosyl Caffeic Acid |
325 |
183 |
- |
7 |
Phenolic Acid |
195 |
- |
- |
8 |
Phenolic acid derivatives |
133 |
- |
- |
Table 1 Proposed compounds found in the crude extract obtained from the leaves of P. edulis.
Property |
QF |
QEPE |
Thickness (mm) |
0.035±0.007 |
0.068±0.005 |
Moisture content (%) |
13.98±0.1169 |
28.27 +1.54 |
Solubility (%) |
49.73±0.001 |
64.58± 0.006 |
Swelling ratio (%) |
85.42±5.861 |
88.59+6.87 |
WVP (g.mm/m2.day.kPa) |
3.02±0.293 |
4.14±0.163 |
Tm (°C) |
119.5 |
120.3 |
ΔH (J/g) |
482.7 |
431.4 |
Xc (%) |
51.02 |
27.89 |
Table 2 Properties of chitosan membrane (QF) and chitosan membrane incorporated with extract obtained of P.edulisleaves.
Water vapor permeability can be related to the crystallinity of the membrane. In general, crystallization of polymers improves barrier properties as a result of the increased packing of the chains, reducing the empty spaces from where gas molecules can pass through. According to the thermal analysis, no significant difference was observed for melting temperature of the chitosan membrane after the incorporation of the extract (Table 2). However, a reduction was observed in the melting enthalpy, and hence in the degree of crystalline of QEPE (Table 2). This can be attributed to the presence of phenolic compounds (large groups) in the extract that difficult the organization of the polymeric chains due to the steric effects. Therefore, more empty spaces are available among the polymer chains, which reduce the barrier property of the membrane and facilitate gas exchange across the membrane.
The chemical interaction of Passiflora edulis extract (EPE) with chitosan and their individual contributions for functional groups were analyzed by infrared spectroscopy. Figure 2 shows the spectra for (a) EPE, (b) QF and (c) QEPE. Table 3 summarizes the main attributions found for each material (EPE, QF and QEPE).For all the spectra, the band between 3600-3200cm-1 indicates the presence of -OH group from water molecules, and the peaks around 2960cm-1are attributed to C–H stretching in organic compounds.16 For EPE spectrum (Figure 2a), the absorption band of C=C bond in the ring (1576-1560cm-1) indicates the presence of aromatic group in the extract. The peaks around 1200–1000cm-1 are attributed to C–O– bond in alcohol and ethers.17 The bands between 1310 and 1390cm-1 can be attributed to the C–O–H angular deformations of phenols, characteristic group of flavonoids.18 The bands between 1576-1560 and 1387/1396 are identification bands of flavonoids and phenolic acid derivatives.19,20 In Figure 2b, the band at 3251cm-1in the spectrum of QF is attributed to the asymmetric stretching vibrationof -NH bonds in amino groups present in chitosan molecules. The bands between 2921 and 2875cm-1 are the secondary bandassigned to C-H stretch vibrations. The peaks at 1637cm-1, 1542cm-1 and 1319cm-1refer to C=O stretching in amide I, N-H flexion (amide II) and C-N elongation (amide III), respectively. These amide bonds in chitosan membrane spectrum are due to the partial deacetylation of chitin to produce chitosan. The band at 1378 cm-1is attributed to acetamide groups, indicating again that chitin was not completely deacetylated (degree of deacetylation of chitosan used> 75%).The band at 1406 m-1refers to carboxylate groups possibly from residual acetic acid used as solvent for chitosan in the preparation of QF. Bands between 1200cm -1 and 1000cm-1 are related to C-O and C-C bonds that correspond to vibrations originating from saccharide structures. Finally, by comparing the FTIR spectra of the membranes, the one incorporated with Passiflora edulis extract (QEPE) shows characteristic bands found for EPE and QF spectra. The presence of characteristic peak of phenolic compounds (1560cm-1) observed in QEPE but not found in QF, confirms the incorporation of the extract in the chitosan matrix (Figure 2c).
Attribution |
Wavelength (cm-1) |
QF |
EPE |
QEPE |
O-H |
3475-3150 |
3251 |
3260 |
3255 |
C-H |
2900-2855 |
2921-2875 |
2960 |
2875 |
C=O (amide I) |
1640-1620 |
1637 |
- |
1654-1615 |
C=C |
1600-1475 |
- |
1576-1560 |
1560-1507 |
N-H (amide II) |
1542 |
- |
1541 |
|
C-O-H |
1440-1220 |
1406 |
- |
1405 |
C-H3 |
1375 |
1375 |
1387/1396 |
1375 |
C-N |
1319 |
1319 |
- |
- |
C-O |
1200-100 |
1061/1023 |
1077/1040 |
1062-1019 |
Table 3 Main attributions found for the extract EPE, and for the membranes QF and QEPE.
In summary, our previous studies on chitosan membranes have demonstrated that they are non-cytotoxic to Vero cells, and improved in vitro wound filling rate of human MG-63 cells.21 Moreover, the results on our in vivo wound healing study using an equine model indicated an intensification on the formation of granulation tissue in skin wounds treated with chitos an membranes.22 Therefore, we expect that association of anesthetic, antioxidant, and antimicrobial activities of Passiflora edulis extract to chitosan membrane has potential for wound dressing application, and would improve skin wound healing as well.9,11,12, 23–28
It was possible to identify the presence of flavonoids in the extract of leaves of P. edulis using LC-Mass and Infrared Spectroscopy. These compounds have antioxidant activity and other properties that would facilitate wound healing. The extract was incorporated in chitosan membranes that demonstrated fluid absorption capacity, water vapor permeability and thermal stability under physiological conditions. Therefore, QEPE showed great potential for application in the treatment of skin wounds. Additional in-vitro and in-vivo wound healing studies are being carried out with P. edulis incorporated in chitosan matrixes. These results will be presented in a further publication.
The authors thank Prof. Marcelo Polo at the Federal University of Alfenas (Unifal-MG) for identifying the P. edulis samples; and Dr. Fernanda Borges de Araujo Paula and Bruno Cesar Correa Salles for providing the extract. The authors also thank Dr. Wagner Vilegas and Dr. Claudia Quintino da Rocha at the Natural Products Bioprospecting Laboratory of the São Paulo State University (UNESP) - Experimental Campus of the Litoral Paulista (São Vicente-SP) for the collaboration on LC-Mass analyses.
This study was inanced in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES)– Finance Code 001.
Declare if any conflict of interest exists.

©2019 Melo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.