Advances in
eISSN: 2572-8490


Research Article Volume 9 Issue 1
Correspondence: Hathama Razooki Hasan, University of Baghdad, College of Science, Department of Chemistry, Iraq
Received: July 17, 2023 | Published: August 8, 2023
Citation: Hasan HR, Hwaidi AQ. Assessment of total peroxidase activity and its relationship with vitamin D in Iraqi rheumatoid arthritis patients. Adv Tissue Eng Regen Med Open Access. 2023;9(1):13-17. DOI: 10.15406/atroa.2023.09.00135
Objective: In a previous work carried on in our laboratory, a positive correlation were recorded between different levels of vitamin D and the antioxidant status in Iraqi patients with rheumatoid arthritis, meanwhile some of peroxidases family have been known to act as antioxidant. Therefore in order to look for the molecular level of this action of vitamin D, the impact of different vitamin D levels on peroxidases activity in Iraqi rheumatoid arthritis patients was investigated.
Method: The study included 119 patients with rheumatoid arthritis (who were matched in age range and body mass index range). Vitamin D was measured by Roche Elecsys vitamin D total II assay, total peroxidase activity was measured by Song's method and total protein was determined by biuret method.
Results: The studied participants in this study were divided into four groups according to their vitamin D levels into: G1 (n=31), G2 (n=30), G3 (n=30) and G4 (n=28), through -out the present study group G4 was used as a control for the comparison purpose. The results indicated that total peroxidase activity and specific activity were highly significantly increased (p<0.001) in patients with severe deficient vitamin D level (G1), and were significantly increased (p<0.05) and highly significantly increased (p<0.001) respectively in patients with deficient vitamin D level (G2). While peroxidase activity and specific activity did not show a significant increase (P>0.05) in patients with insufficient vitamin D level (G3).
Conclusion: In rheumatoid arthritis patients, vitamin D level affects peroxidases activity, as concluded from the negative counterbalance relationship that was found between the level of vitamin D and peroxidases activity.
Keywords: rheumatoid arthritis, vitamin D level, total peroxidase activity
RA, rheumatoid arthritis; ACPA, anti-citrullinated protein antibodies; ROS, reactive oxygen species; OS, oxidative stress; CRP, C-reactive protein
Rheumatoid arthritis (RA) is an inflammatory autoimmune disease.1 It can quickly develops with a short time into severe disease.2 The synovial joints are the main joints that are affected by this disease and the patients suffer from pain, and loss of their joints function.3 In this disease rheumatoid pannus (a type of extra growth in the joints that can cause pain, swelling, and damage to the bones, cartilage, and other tissue) and autoantibodies, like anti-citrullinated protein antibodies (ACPA) and rheumatoid factor (RF) were produced by inflamed synovial joint and thus lead to damage of cartilage and bone.4
Although the exact cause of this disorder remains unknown, there is evidence suggesting that reactive oxygen species (ROS) generated within the body play a significant role in rheumatoid arthritis (RA).5 In individuals with this disease, ROS may be produced by chondrocytes and activated neutrophils within the affected joints.6 Normally, the human body possesses an antioxidant defense mechanism designed to protect against the harmful effects of ROS, known as the anti-oxidative system.3
Oxidative stress (OS) is a term applied to the cell as a result of presence of excess oxidants that accompanied with antioxidants deficiency7 and the OS result from the disruption of the balance between body’s oxidants load and antioxidants reservoir.8
Among the important enzymes involved in OS is total peroxidase (EC 1.11.1.X), which is a large family of iso-enzymes present in almost all living organisms. It has been reported that total peroxidase has roles in both human pathologies and disease prevention.9 Two major super- families of peroxidase enzymes exist in the human body: the first one contains iron and exists as heme peroxidase. It produces free radical by one electron transfer as illustrated below:10,11
Heme peroxidase can react with hydrogen peroxide and produce oxidized enzyme that then oxidizes a substrate.12,13 This type of enzyme differs in their oxidizable substrate specificity.14
The second type of superfamily is the peroxidase with alternative catalytic site that contain selenium and sulfur.14 This type plays an important role as antioxidant and tends to catalyze two electron transfer as shown below:
This type of peroxidase lower the level of hydrogen peroxidase without forming any free radicals intermediates, by doing so, it acts as antioxidants.
Vitamin D, a fat soluble vitamin is involved in regulation of calcium and phosphorous homeostasis and bone metabolism.15 Recently, a non-calcemic function has been reported for this vitamin in many disease and its deficiency has been mentioned to be related with several autoimmune disorders like RA.16 A previous work which was done in our laboratory has confirmed the role of vitamin D as an antioxidant in RA patients.10 Since, up to the best of our knowledge no study has been found in the literature that explore the peroxidase activity in rheumatoid arthritispatients, the goal of this study is to evaluate the activity of peroxidase in the patients with this disease and to study the correlation between peroxidase activity and the different levels of vitamin D in the serum of RA patients.
Patients
A total number of 119 patients was included in the present study. They were Iraqi rheumatoid arthritispatients, their age were 40-60 years (mean±SD=50.01±5.56) with the same body mass index range. All patients were medically diagnosed and had a positive C-reactive protein (CRP). They were attending different hospitals and medical centers in Iraq. Patients with chronic renal disease, systemic lupus erythematosus, diabetes mellitus, or any other systemic disorder and those who were smokers, or alcohol drinkers were excluded from this study, as were those taking enzyme inducer drugs or calcium and vitamin D supplements. The protocol of the present study was approved by the College of Science / University of Iraq / Ethics committee.
Biochemical measurements
Fasting blood samples were taken from rheumatoid arthritispatients, then the sera were separated and stored at -20ºC until been used. Determination of vitamin D level was performed using Roche Cobas e 411 analyzer and Roche Elecsys vitamin D total II assay (Roche Diagnostics, Mannheim, Germany). Elecsys vitamin D assay usesvitamin D binding protein to react with 25-hydroxyvitamin D3 and D2. The level of vitamin D in human can be defined as severe deficient (where the level≤10 ng/mL), deficient (where the level = 11–20 ng/mL), insufficient (where the level=21–30 ng/mL) and sufficient (where the level>30 ng/mL).17,18
Peroxidase activity was analyzed by applying a colorimetric method that based on using phenol, 4-aminoantipyrine, and H2O2 as the dye-generating compounds.19 The enzyme activity is expressed as the increase in absorbance at nm resulting from the decomposition of hydrogen peroxide per time of incubation (ΔA/min). The peroxidase specific activity is expressed as the unit of the enzyme activity per gram of protein.
Determination of protein concentration in the collected sera samples was done using biuret method20 in which bovine serum albumin (BSA) was used as a standard. Discontinuous polyacrylamide gel-electrophoresis was carried out by Polyacrylamide gel (separating gel 7.5% total monomer and 2.6% cross linker) was prepared according to native PAGE Laemmli’s gel system21 and 4-aminoantipyrine stain was used to detect the location of the peroxidases activity on the gel.19,22
The results of this study was expressed in the form of (mean value±the standard deviation). Analysis of the data normality measures were tested with Shapiro-Wilk Test. And the results were analyzed by SPSS version 20 (One-Way ANOVA and Pearson correlation), where the one-way analysis of variance (ANOVA) was used to compare the groups by using the least significant difference as a post hoc test to make individual comparisons. Statistically significant differences were determined by a p<0.05. The difference was considered as highly significant, when (P<0.001), significant when (P<0.05) and non-significant when (P>0.05).
The current work studied population consisted of 119 RA patients whose their ages mean value (50.01±5.56) year. The gender distribution of RA disease among the used samples of Iraqi patients was calculated as percentage (Figure 1).
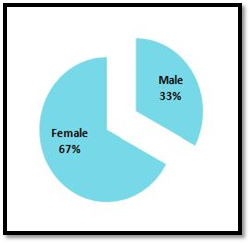
Figure 1 The distribution percentage of the disease according to the gender of the studied rheumatoid arthritis patients.
The results of the determination of peroxidase activity and vitamin D level in the serum of Iraqi rheumatoid arthritispatients involved in this study are shown in Table 1.
Parameter |
n |
Mean±SD |
Ages (year) |
Peroxidase (U/L) |
119 |
31.22±10.47 |
50.01±5.56 |
Vitamin D(ng/L) |
119 |
20.64±11.30 |
50.01±5.56 |
Table 1 The mean value and standard deviation (mean±SD) of peroxidase activity and vitamin D level in the studied patients with their ages range
When the measured level of vitamin D in the patients was examined, the results indicated that the patients enrolled in the current study had different level of this vitamin in their serum, which allowed us to divide them into four groups according to references17,18 and as follows: G1 (vitamin D severe deficient group, n=31), G2 (vitamin D deficient group, n=30), G3 (vitamin D insufficient group, n=30) and G4 (vitamin D sufficient group, n=28) as shown in Table 2. In order to explain the impact of vitamin D level on peroxidases activity present in these patients, G4 was used as a control throughout the current study.
Groups |
n |
Vitamin D (ng/mL) |
Ages (year) |
(G1) |
31 |
6.96±1.72 |
49.80±5.85 |
(G2) |
30 |
15.82±2.54 |
51.30±4.55 |
(G3) |
30 |
24.68±2.49 |
50.40±5.49 |
(G4) |
28 |
36.64±4.18 |
48.46±6.19 |
Table 2 Level of vitamin D (mean value±SD) in the different studied groups with the age’s range of the patients
The gender distribution among the different vitamin D level groups indicates and as Figure 2 shows that the incidence of the disease in Iraqi female is about double as higher in Iraqi male. This is in line with Krause and Makol, who reported that rheumatoid arthritis occurs in female commonly two to three times more than in male in USA.23 Also this result is consistent with that has been reported in another Iraqi study.24
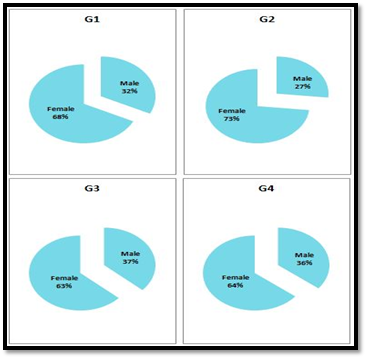
Figure 2 The percentage of gender distribution in each group (G1, G2, G3 and G4) who were classified according to vitamin D level.
The results of peroxidases activity (U/L) measurement and their specific activity (U/g) calculation in serum samples of the four studied groups are presented in (Figure 3&4 respectively).
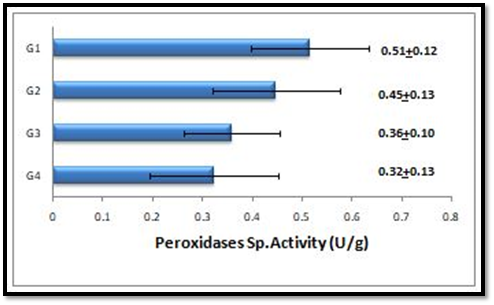
Figure 4 Comparison between the specific activities of peroxidase among the different studied groups.
The results in Figure 3&4 show that the peroxidase activity and specific activity are highly significant increased (p<0.001) in the patients with severe deficient vitamin D level (G1). Meanwhile peroxidase activity and specific activity are significantly increased (p<0.05) and highly significant increased (p<0.001) respectively in patients with deficient vitamin D level (G2). While both of these parameters are observed to be non-significantly increased (P>0.05) in patients with insufficient vitamin D level (G3) in comparison to that of G4. Upon performing Pearson correlation between the levels of vitamin D in each groups (G1, G2, G3 and G4) and their corresponding peroxidases activity. Vitamin D level has a significant negative correlation with the peroxidases activities in all studied patient groups (G1, G2, G3 and G4) (p<0.05) (Figure 5).
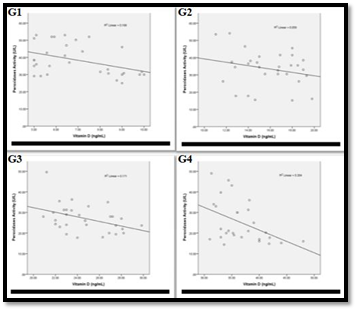
Figure 5 Association of peroxidases activity with vitamin D level in:
G1: The correlation coefficient -0.410 and the correlation is significant (p<0.05)
G2: The correlation coefficient -0.243 and the correlation is significant (p<0.05)
G3: The correlation coefficient -0.413 and the correlation is significant (p<0.05)
G4: The correlation coefficient -0.452 and the correlation is significant (p<0.05)
Figure 6 shows the Pearson correlation between the level of vitamin D in each groups (G1, G2, G3 and G4) with their peroxidases specific activity. As it is clear from this Figure, the results prove that vitamin D level has a significant negative correlation with the peroxidases specific activity in all studied patient groups (G1, G2, G3 and G4).
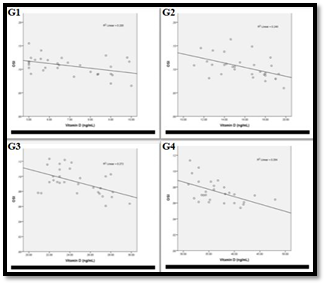
Figure 6 Association of peroxidases sp. activity with vitamin D level in:
G1: The correlation coefficient -0. 447 and the correlation is significant (p<0.05)
G2: The correlation coefficient -0.310 and the correlation is significant (p<0.05)
G3: The correlation coefficient -0.470 and the correlation is significant (p<0.05)
G4: The correlation coefficient -0.488 and the correlation is significant (p<0.05)
From the above results, it is worth to notice that there is a significant negative association between the peroxidases activity and vitamin D levels (Figures 3–6).Mean while the activity of peroxidases in the patients with sufficient vitamin D level (G4) is near to its activity in patients with that of insufficient vitamin D level (G3). This may suggest that peroxidases activity is influenced by a certain level of vitamin D. Such conclusion was confirmed by polyacrylamide gel-electrophoresis (PAGE) 7.5%, which was used to illustrate the differences in the separation profile of peroxidase isoforms in the serum of the different studied groups. The peroxidase activity was detected for the different bands on the gel, by staining the gel using 4-aminoantipyrine stain. Figure 7 shows the electrophoresis pattern of peroxidases after staining for enzymatic activity in these samples (G1, G2, G3 and G4) groups. Where it is clear that when the gel was stained for enzyme activity, different forms of peroxidase are observed to be present in the serum of the four studied groups. These forms are different in their relative motilities, which reflect changes in their molecular weights and charges. Furthermore it is obvious from the comparison of the peroxidase activity profile that the enzyme in the serum of G1 and G2 sample was detected into two separated zones. While in G3 and G4, only one zone could be detected on the gel. The electro- gram shows that the numbers of bands observed in G1 and G2 are equal, meantime that, which observed in G3 and G4 are equals.
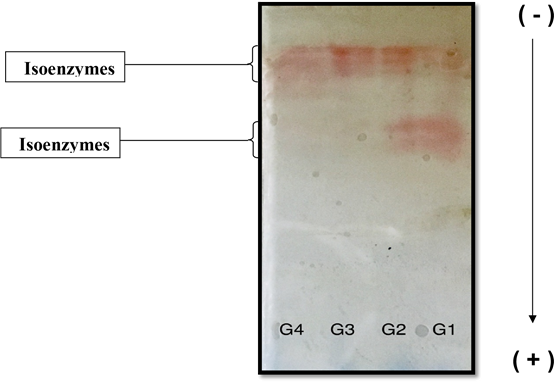
Figure 7 Discontinuous polyacrylamide gel-electrophoresis (PAGE) 7.5%, using Tris -glycine buffer, pH 8.9 as electrode buffer. Electrophoresis was carried out for 3 hours at 4 °C by using a constant current of 40 mA & voltage of 15v/cm. The gel was stained for peroxidases activity stain using 4-aminoantipyrine stain. The samples (18μg) applied were as follows:
G1 Pooled crude serum (Vitamin D severe deficient)
G2 Pooled crude serum (Vitamin D deficient)
G3 Pooled crude serum (Vitamin D insufficient)
G4 Pooled crude serum (Vitamin D sufficient)
The results of the present study are in agreement with our previous study results, which reports that the low level of vitamin D correlate with the presence of increased level of the oxidants.9 Moreover the current results confirm the non-calcemic role of vitamin D in our bodies that has been suggested by9,15,18,23.It is worth to mention that the present study is the first study to address the association between vitamin D status and peroxidases activity in RA patients.
Generally in human body, one of the major sources of ROS is NADPH oxidase family. This family generates superoxide radicals (O2●–) which are the initiator of many ROS, and can be transformed into hydrogen peroxid.23 Hydrogen peroxide is the main in vivo oxidants, which is the main substrate on which peroxidases act.24 The normal level of hydrogen peroxide within plasma is 1–5μM, this level is considered as high as 50μM in the case of inflammatory disorders.25–27 The peroxidase cycle is illustrated in the following equations:
Compound I is not explicit in regards to the one-electron donor; it tends to be exogenous, or endogenous.14 Hydrogen peroxide can be considered as a one-electron oxidant only with myeloperoxidase compound I, with the generation of superoxide.28
The elevation in the peroxidase activity recorded here might be attributed to heme peroxidase such as myeloperoxidase, such increase may cause an increase in the total oxidant status that lead to the reported oxidative stress in RA patients.29 Heme peroxidases in general, can promote, rather than prevent oxidative damage, since the decrease of the oxidized enzymes may achieve by one-electron transitions, this implies production of oxidant from the reducing substrates that can direct to chain reactions.10
The antimicrobial activity of human peroxidases is attributed to their ability to produce cytotoxic hypohalous (HOX, X= Cl–, Br–, and I–) and hypothiocyanous (HOSCN) acids.29–32 HOCl and HOBr are extremely strong oxidants that have diffusion-controlled reactions with biomolecules. They disperse just a short distance and react nonspecifically with many molecules, leading to substantial harm within a small radius of their production site, as with any strong oxidant.33 While HOSCN is a reasonably long-lived oxidant that reacts primarily with thiol groups due to its lower reduction potential. It can pass a peroxidase's oxidative capacity over longer distances with less harm to host tissues.25
Vitamin D has been suggested to exhibit its antioxidant activity via modifying some of the antioxidant enzyme.15 Our findings, here, indicate a potential association between vitamin D levels and peroxidase activity, as well as specific activity. This suggests that vitamin D may act as an antioxidant by influencing the gene expression of heme peroxidases, such as myeloperoxidase. However, further confirmation of these conclusions is necessary through larger-scale studies involving more participants. Based on our results, it is advisable for rheumatoid arthritis patients to consider taking vitamin D supplements. This could potentially enhance the efficiency of antioxidants and restore the balance between oxidants and antioxidants, thereby reducing oxidative stress previously reported in these patients.9 Furthermore, it may contribute to slowing down the progression of the disease and alleviating the associated pain.
None.
The authors declare that there are no conflicts of interest.
None.

©2023 Hasan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.