Advances in
eISSN: 2572-8490


Mini Review Volume 4 Issue 4
Biotechnology Department, University of Manouba, Tunisia
Correspondence: Mohamed Neifar, LR Biotechnology and Bio-Geo Resources Valorization (LR11ES31), Higher Institute for Biotechnology, University of Manouba, Biotechpole of Sidi Thabet, 2020, Ariana, Tunisia, Tel 7052 7882
Received: June 16, 2018 | Published: August 22, 2018
Citation: Neifar M, Ouertani R, Askri R, et al. Implantable enzymatic fuel cells: opportunities and challanges. Adv Tissue Eng Regen Med Open Access. 2018;4(4):87-89. DOI: 10.15406/atroa.2018.04.00083
The generation of electrical power using enzyme biocatalysts has recently received great attention due to the increased demand for implantable medical devices. Enzymatic fuel cells hold great potential as a sustainable means to power micro-scale and nano-scale devices by using blood glucose as a fuel and dissolved oxygen as an oxidizer. Here, we summarize the current state of implantable enzymatic fuel cells with emphasis on engineering aspects, device performance and future prospects of this biological battery technology in biomedicine.
Keywords: biocatalysts; enzymatic fuel cells; Self-powered implantable electronics; biomedicine; green energy
EFC, enzymatic fuel cells; DET, direct electron transfer; MET, mediated electron transfer
Enzyme-based biofuel cells
Implantable enzymatic fuel cells (EFCs) utilize microbial oxidoreductases as biocatalysts for in vivo power generating through the electro-catalytic oxidation of glucose to gluconolactone at the anode and the reduction of dissolved oxygen to water at the cathode.1 These extracellular enzymes display high activities at physiological pH and room temperature and could be produced by selected microorganisms at a low cost.2 No artificial fuel and oxidant are needed to achieve EFC performance.3,4 EFCs are highly promising due to an array of desirable technological criteria such as efficiency, selectivity and biocompatibility of the enzymes and therefore, they could be used to power implantable medical devices.4‒9 In the last two decades, significant progresses in EFC technology have been made and when considering implanted medical electronics, the use of functional glucose/O2 implantable EFCs (Figure 1) has received a great deal of attention.3,4
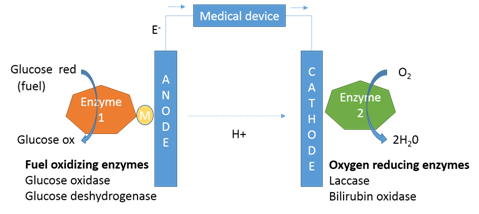
Figure 1 Schematic of an implantable enzymatic fuel cell. The anode enzyme comprises immobilized glucose oxidase or glucose deshydrogenase and the cathode enzyme comprises immobilized laccase or bilirubin oxidase. Generally, glucose is indirectly oxidized (through a redox Mediator) at the surface of the anode and oxygen is directly reduced at the surface of the cathode. The coupled glucose oxidation/oxygen reduction reactions provide a self-generating current source to biomedical devices.
Schematic of an implantable enzymatic fuel cell (Figure 1). The anode enzyme comprises immobilized glucose oxidase or glucose deshydrogenase and the cathode enzyme comprises immobilized laccase or bilirubin oxidase. Generally, glucose is indirectly oxidized (through a redox Mediator) at the surface of the anode and oxygen is directly reduced at the surface of the cathode. The coupled glucose oxidation/oxygen reduction reactions provide a self-generating current source to biomedical devices.
The implanted EFC systems are characterized by an extremely simple design in which enzymes are employed for glucose oxidation at anodes and O2 reduction at cathodes2 (Figure 1). The reference electrode and its calibration are not necessary in such design contrary to other electrochemical sensors.4 Due to the abundance of sugars and the availability of O2, the sugar-oxidizing and O2-reducing oxidoreductases are considered as suitable and attractive enzymes for the construction of implanted EFCs.1,5‒9 These enzymes are connected to the electrode surface via direct electron transfer (DET) or mediated electron transfer (MET).1‒3 The current advance in EFC technology today is the construction of bio-electrodes (cathodes and anodes) that use DET instead of MET. Indeed, the direct transfer of the electrons from the enzymes to the electrode avoids the toxicity problem associated with the use of redox mediators such as osmium-based mediators.10‒13
The most studied anodic enzyme is glucose oxidase, which can directly or indirectly (via mediator) electro-oxidize glucose. However, the use of this enzyme in implantable conditions encountered some problems such as the generation of a toxic by-product (H2O2) and the need of supplementary adjustments of the enzymatic devices for their in vivo operation.1,2 In order to avoid such problems, other glucose-oxidizing enzymes, that not generate hydrogen peroxide in implanted environment, can be used such as the NADH-dependent glucose dehydrogenases. The bio cathodes of implanted EFCs are generally operated with enzymes that catalyze the direct oxygen reduction to water such as blue multicopper oxidases including laccase, ceruloplasmin, and bilirubin oxidase and ascorbate oxidase.1‒4
Implanted biomedical microsystems (cochlear, retina implant and glaucoma sensor, intracranial pressure sensor, artificial sphincters and sphincter sensors, etc.) are promising tools used in medical and engineering disciplines.10 Numerous examples of potentially interesting implantable EFCs (pacemakers, neurostimulators, drug carriers, etc.) have been reported. Despite the lower power produced by implantable EFCs, they can provide the required energy inside living organisms.1‒4
The first glucose/oxygen EFC implanted in a living animal was described by Cinquin et al.14 The EFC consists of an anode and a cathode composed with immobilized glucose oxidase and laccase, respectively. This EFC generated a stable power (6.5µW at 0.13V) inside the rat for 3months without significant inflammatory signs. However, the existence of membranes and dialysis bags limits the practical applications of such implanted EFC system. Sales et al.,15 also monitored and controlled a glucose/oxygen EFC implanted in a living rat. The EFCs produced an open circuitry voltage and a maximum power density of 125mV and 95μW cm-2, respectively. El Ichi-Ribault et al.,16 tested a glucose/oxygen EFC implanted in a rabbit for 2months. The electronic bio device delivered continuously 16 μW mL−1 during 16days. The power output decreased at the end of the in vivo implantation period due to inflammatory processes.
The first EFC tested in humans in non-invasive (ex vivo) contact conditions, is described by Pankratov et al.,17 who tested a cellobiose dehydrogenase-bilirubin oxidase EFC in flow-through mode during 10minutes by continuously drawing human blood. The EFC produced enough energy to power a low voltage medical device. Southcott et al.,18 described a pacemaker powered by a glucose oxidase-laccase implantable EFC assessed under conditions mimicking the human venous blood circulatory system. The EFCs produced an open circuitry voltage and a short circuitry current of 470mV and 5mA, respectively. The designed electronic implantable medical device generated a profile of electrical pulses very similar to those registered with a commercial device. A number of interesting examples of implantable EFCs have been reported and reviewed by Ganzales-solino,3 Onuki et al.,6 Kotanen et al.,7 and Barton et al.9
Despite the progress that has been made in the last decade, many challenges remain for practical applications of implantable EFCs. First limitation is the low stability of the used enzymes compared to the average lifetime of a lithium battery-based implanted device.3,4,9 Enzyme based catalysts have typically short lifetimes (several hours) in solution. Biocatalyst immobilization on electrode surfaces by entrapment, chemical bonding and cross linking extended enzyme lifetimes to several days. Recently, enzyme active lifetimes reached about 1year through encapsulation as biocatalyst immobilization technique.13,19 The host immune response observed after implantation process is considered as another challenge for in vivo application of EFCs. In fact, the living organism reacts to unfamiliar materials through a cascade of immune reactions leading to the formation of a collagen capsule around the EFC device decreasing the analyte access.19 Moreover, proteases present in tissues could degrade partially or completely the immobilized enzymes.20 On the other hand, the complexity of the blood matrix, which contains cells and different biomolecules that can precipitate onto the electrode surface and interfere with the electron transfer and/or inhibit the used enzymes.3,4,9 Recent advances in nanotechnology and micro engineering and a better understanding of the EFC bio- processes certainly helped the development of composite nonmaterial’s that can be easily integrated into implanted EFCs for future biomedical purposes.3-7,11 To confront the challenges associated with implantable EFCs, several wearable EFCs exploring physiological fluids (saliva, transdermal fluid, sweat, tears, urine, etc.) as an alternative to blood, have been recently applied to power a number of medical devices.3,4
None.
The author declares no conflict of interest.

©2018 Neifar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.