Advances in
eISSN: 2572-8490


Mini Review Volume 4 Issue 2
LR Biotechnology and Bio-Geo Resources Valorization, University of Manouba, Tunisia
Correspondence: Ameur Cherif, LR Biotechnology and Bio-Geo Resources Valorization (LR11ES31), Higher Institute for Biotechnology, University of Manouba Biotechpole of Sidi Thabet, 2020, Sidi Thabet, Ariana, Tunisia, Tel 70 527 882
Received: July 27, 2017 | Published: March 15, 2018
Citation: Neifar M, Naili F, Chouchane H, et al. Potential use of microbial thermo-bioplastic polyhydroxyalkanoate as promising tissue engineering biomaterial in biomedicine. Adv Tissue Eng Regen Med Open Access. 2018;4(2):35-38. DOI: 10.15406/atroa.2018.04.00074
Biologically-synthesized plastic, polyhydroxyalkanoates (PHAa), have been attracting interest in biomedecine due to its similar physical properties to synthetic plastics. Due to its biocompatibility and biodegradability, poly(3-hydroxybutyrates) (PHBs) and related copolymers, mostly poly(3-hydroxybutyrate-co-3-hydroxyvalerates) (PHBVs) has been widely evaluated as carriers for drug delivery or scaffolds in a wide variety of tissue engineering applications. Screening and selection of novel suitable (extremophilic) bacterial strains, low-cost renewable carbon sources, efficient fermentation technologies of natural and recombinant microorganisms and PHA recovery processes are very interesting aspects that should be taken into consideration for the commercialization of microbial PHAs with industrial potential. This review aims to illustrate the start-up of PHA research in biomedicine by providing a summary of their unique physiochemical and mechanical properties for use as biomedical materials for bone, cartilage, cardiovascular, nerve conduit, skin and esophagus tissue engineering.
Keywords: bioplastics, PHA-producing bacteria, extremophiles, tissue engineering, biocompatible, biomedicine
The potential of bio based plastics for replacement of petrochemical plastics is 90%, corresponding to 240 million tons per year.1 Bio-based materials such as polynucleotides, polyamides, polysaccharides, polyoxoesters, polythioesters, polyanhydrides, polyisoprenoids and polyphenols are potential candidates for substitution of synthetic plastics.2 Polyhydroxyalkanoates (PHAs), belonging the group of polyesters, have been received a great deal of attention because they possess biodegradable thermoplastic properties.3
PHAs are synthesized by microorganisms under unbalanced growth conditions. They act as ideal storage compounds due to its insolubility inside cytoplasm, which exert negligible increase in osmotic pressure.4 PHA producing microbes as storage biomaterials are able to survive during starvation period compared to those non PHA-producing microbes as these energy-reserve biomaterials slow down the cell autolysis and subsequently its mortality.5 Among the more than 300 PHA-producer strains, only a limited number of microorganisms have been employed for the biosynthesis and commercialization of PHAs including Alcaligenes latus, Cupriavidus necator, Pseudomonas oleovorans and Bacillus megaterium, which are able to grow on different low cost carbon sources including plant oils or wastes to produce PHA.6 Some bacteria have been described as producer of PHA as much as 90% (w/w) of dry cells during depletion of essential nutrients such as nitrogen, phosphorus or magnesium.7 A vast number of PHAs from extremophiles were reported over the last two decades (Table 1). For examples, the highly halotolerant bacterium Halomonas boliviensis LC1 could grow and produce 56% CDM of scl-PHA P3HB from starch hydrolysate under saline conditions (0.77 M NaCl) while the thermophilic bacterium Thermus thermophilus HB8 synthesized up to 35.6% CDM of scl-mcl-PHA copolymer from whey at a high cultivation temperature of 70°C.8,9
Microorganisms |
Sample nature |
Extremophily |
Carbon source |
Monomere/polymere PHA / PHA nature |
Ref. |
Halogeometricum borinquense (TN9) |
brine and sediment samples of solar salterns of India |
20 % NaCl |
Glucose |
P3HB (thermostable) |
|
Halogeometricum borinquense (E3) |
Tuzkoy Salt Mine (TK) |
25% NaCl |
Corn starch |
P(HB-co-HV) |
|
Halomonas boliviensis |
Skin of the red sea squirt Halocynthia papillosa |
12% Nacl pH 8.0-10.0 |
Starch hydrolysate |
P3HB |
|
Thermus Thermophilus |
- |
65°C |
sodium gluconate |
scl-mcl- PHA (thermostable) |
|
Thermus thermophilus HB8 |
|
60-75°C |
|
P3HB (thermostable) |
|
Pseudomonas sp. SG4502 |
- |
55°C |
Glucose |
P3HB |
|
Pseudomonas oleovorans |
- |
- |
fatty acid |
mcl-PHA |
|
Bacillus cereus PW3A |
polluted water sample |
- |
Glucose |
P3HB (thermal and mechanical properties) |
|
Bacillus Megaterium |
Yufutsu gas field |
40°C |
Glucose |
3HB, 3HV, 3HHx |
|
Cupriavidus necator H16 (thermophile) |
Hot spring in Southern Taiwan |
50°C |
Glucose |
Thermostable PHB |
Table 1 Some extremophilic PHA-producing microbial strains
The manipulation of natural and recombinant microbial producers of PHA to achieve high PHA production has been the subject of many studies.4,7 E. coli has been the selected host for genetic techniques devised to introduce the PHA biosynthetic genes, improve their expression, provide suitable quality and concentration of substrates to the PHA synthase, as well as to modify the host strains to improve their performance in the bioreactor.22 Nowadays, microbial PHAs continue to attract increasing industrial interest as renewable, biodegradable, biocompatible, and extremely versatile thermoplastics.23 Microbial PHAs are the only waterproof thermoplastic materials available that are fully biodegraded in aerobic and anaerobic environments. There are according to their monomer composition: short-chain length (SCL) PHAs and medium-chain length (MCL) PHAs. SCL-PHAs are polymers of 3-hydroxyacid monomers with a chain length of 3 to 5 carbon atoms, such as poly(3-hydroxybutyrate) (PHB, the most common PHA); whereas MCL-PHAs contain 3-hydroxyacid monomers with carbon atoms between 6 to 16. All of them are optically active compounds.24 Around 200 different monomer constituents were found in the polymers.25 This versatility is due in particular to the wide substrates of the PHA-synthesizing enzymes, and gives PHAs an extended spectrum of associated properties which is a clear advantage compared to other bioplastics. PHAs are biocompatible and for this reason they have also attracted attention as raw material to be used in medical devices.26 PHA has been generally manufactured for polymer films, non-woven materials, and pharmaceutical products used in transplantology, surgery, and in tissue engineering.27 Although PHAs have the potential to be used as tissue engineering biomaterials, only a few of them have been produced industrially.28,29 Nowadays PHAs types such as PHB, PHBV, and PHBHHx are used as substitutes for cardiovascular tissue, heart valve tissue, nerve conduit tissue, skin tissue, subcutaneous tissue, esophagus tissue, cartilage tissue, and bone tissue (Figure 1).
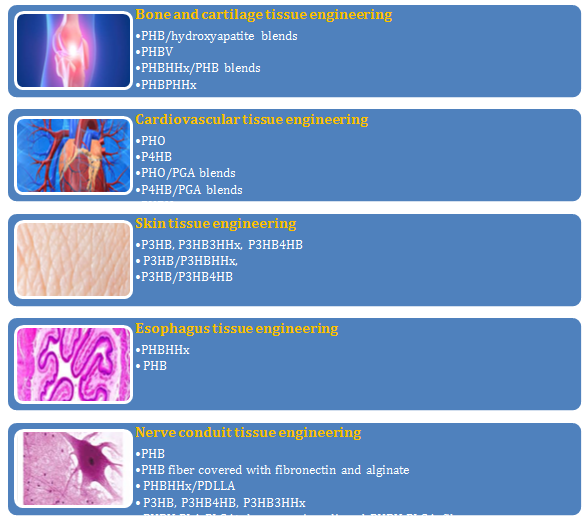
Figure 1 Tissue engineering applications of PHAs.22
In tissue engineering, the cells are grown in vitro on biopolymers to construct tissue for implantation purposes.30 A high level of biocompatibility is usually needed before foreign biomaterials can be incorporated into human body. Shape, surface porosity, chemistry of the biomaterials and the environment of the tissue play important roles in biocompatibility.31‒33 PHAs have shown good potential as medical implant biomaterials. For examples, Neural stem cells (NSCs) grown on/in PHA scaffolds may be useful for repairing central nervous system (CNS) injury.34 Among the PHA family members, PHBHHx appears to have the strongest potential to promote NSC differentiation into neurons. It has been suggested that PHBHHx nanofiber scaffolds that promote NSC growth and differentiation could be developed for treating CNS defects.
Cells for bone and cartilage regeneration have been also evaluated for their proliferative and differentiative responses when inoculated into PHBHHx-based scaffolds.35,36 It has been reported that PHBHHx scaffolds increase both chondrocyte proliferation and protein secretion in rabbit articular cartilage cells seeded onto them.37,38
Considering that its strength and elastic properties can be adjusted by changing its monomer contents, PHBHHx could be tailored to meet the requirements for regenerating both bone and soft tissues. PHBHHx, with its excellent mechanical and thermal properties, has been used as a scaffold for tissue-engineered cardiovascular products.39,40
In conclusion, microbial PHAs offer great practical potential as tissue engineering biomaterials. New targets and strategies for the improvement of PHA production will certainly be developed in the next future, including screening and selection of novel extrophilic PHA producers and tailor-made PHAs with desired monomer compositions. In order to design a completely sustainable PHA production process, strains should be further metabolically engineered to produce PHAs up to sufficiently high polymer content with high productivity from the most inexpensive carbon source through fine-controlled fermentation schemes. With novel composite material generation coupled with technical innovations, microbial PHAs may be of clinical benefit in regenerating a variety of tissues.
None.
The authors declare no conflict of interest.

©2018 Neifar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.