Advances in
eISSN: 2572-8490


Mini Review Volume 3 Issue 3
1Department of Neurosurgery, Hospital Celso Pierro, Brazil
2Department of Neurosurgery, Hospital Santa Paula, Brazil
3Department of Neurosurgery, Hospital AC Camargo Cancer Center, Brazil
4Department of Neurosurgery, Hospital Ramos Mejía, Buenos Aires University, Argentina
Correspondence: Maick Willen Fernandes Neves, Medical Resident, Department of Neurosurgery, Hospital Celso Pierro, PUC Campinas, Campinas, AvenidaIbijaú 368, Moema, São Paulo, SP, Brazil, CEP 04524-02, Brazil
Received: November 17, 2017 | Published: December 29, 2017
Citation: Neves MEF, Aguiar PHP, Belsuzarri TAB, et al. Anterior pontine cavernoma: what should be done? Adv Tissue Eng Regen Med Open Access. 2017;3(3):403–406. DOI: 10.15406/atroa.2017.03.00067
Introduction: Anterior pontine cavernomais a rare pathology with difficult access; it predominantly occurs in middle-aged patients with a high risk of bleeding and rebleeding. There is a direct association with genetic inheritance due to the CCM1, CCM2 and CCM3 genes, and if it is associated, there is a higher risk of severity.
Materials and methods: Analysis of PUBMED publications about ventral pontine cavernomas, anterolateral pontine cavernomas, and main surgical approaches.
Results and discussion: Pontine cavernomas have a bleeding risk of 5% per year and rebleeding of 30% per year; therefore, it is a surgical pathology in which most authors approach from four to six weeks after bleeding for total resection and curative purposes. There are different approaches and they depend on the topography of the lesion and the surgeon`s ability; technologies such as monitoring, neuronavigation, and tractography must be used to reduce the risk of lesions. The most used approaches are retrosigmoid, presigmoid, and anterior transpetrosal; the use of endoscopic approaches has been increasing recently.
Conclusion: Anterior pontine cavernoma is a severe pathology that must be surgically treated to decrease the risk of bleeding and rebleeding.
Keywords: cavernoma, pontine cavernoma, cavernous angioma, pontine hemorrhage
Cavernoma is the most common vascular malformation of the brain with an incidence of 0.4-0.5%, prevalence of 0.6 per 100,000 inhabitants, predominance in males between 20-40 years old, and predominance of 25% in children.1–4 Brainstem cavernomas are rare (18%) and present a bleeding rate higher than supratentorial cavernomas; they are more common in the pons (61%), with an estimated bleeding rate of 2-6.6% per year.1,5–8 In the pediatric population, the lesion is found in the pons (rate of 73%), and it presents a 12.3% rate of bleeding per year and a 32.5% rate of rebleeding per year.9
There is a genetic inheritance relationship, autosomal dominant, associated to the CCM1 gene (cerebral cavernous malformation 1), CCM2, and CCM3.2 CCM1 is located on chromosome 7 at band 7q11.2-q21, CCM2 is located at band 7p15-p13, CCM3 is located on chromosome 3 at band 3q; 40% are located in mutation 1 and 20% in mutation 2.4 When it is related to family inheritance, there is a higher chance of multiple cavernomas and a higher rate of hemorrhage.2
Articles were searched on PUBMED and they were based on the treatment of ventral pontine cavernomas and anterolateral pontine cavernomas; these articles were critically analyzed and tables were constructed for data analysis.
The diagnosis is performed by magnetic resonance imaging (MRI) and clinical presentation; the most common symptoms are hemorrhage, seizure, gaze palsy, diplopia, obstructive sleep apnea, vertical and torsional eye movements, gait disturbance, headache, and focal deficit. Cavernomas can also be diagnosed unexpectedly.1,4,10–13 The risk of bleeding ranges from 5% to 6.6% per year and the risk of rebleeding ranges from 26% to 30%.1,8 Therefore, the treatment should always be performed in adults. In the pediatric population, the indication includes multiple hemorrhages, lesions equal or bigger than 2cm, severe or progressive deficits, exophytic lesions, and mass effect on the brainstem.9
The treatment of choice is surgery, 4-6 weeks after bleeding, although the time is still controversial because the approach is easier in the period that the hematoma is not yet organized; in this case, the goal is the complete removal, preventing recurrence of bleeding and functional worsening.1,14,15 However, before indicating the surgical procedure, the main differential diagnosis (pontine glioma and multiple sclerosis) must be excluded.16
Brainstem cavernomas located on the pons are classified in dorsal, lateral, and ventrolateral.17 Accesses to approach the anterior region of the pons are dangerous due to the risk of corticospinal tract lesions and worsening of postoperative deficits. Anterolateral accesses have been used for ventromedial lesions due to the corticospinal tract in the ventromedial region of the pons.18 Peng Hu et al.17 observed that the retrosigmoid approach was mostly chosen in 83 patients with cavernomas in the ventrolateral region of the pons (Table 1).
Retrosigmoid approach |
49,4% |
Presigmoid approach |
13,3% |
Subtemporal approach or sub temporal combined with suboccipital approach |
13,3% |
Transpetrosal approach |
9,6% |
Table 1 Surgical approaches in the ventrolateral region of the pons
Gross et al.19,20 performed a meta-analysis with 1390 patients and obtained a total resection rate of 91% and a mortality rate of 1.5%. Another meta-analysis performed by the same authors with 683 patients showed a worsening of neurological deficits in 14% of the patients and a mortality rate of 1.9%. In the first analysis, the following approaches were observed for cavernomas of the anterior part of the brainstem: retrosigmoid, anterior transpetrosal (Kawase), and presigmoid/retrolabyrinthine.
The size of the cavernoma or hematoma is not associated with surgical success and the hematoma facilitates dissection; however, when bleeding occurs again, the dissection is difficult due to the formation of a fibrous capsule.21 The entry region in the anterolateral part of the pons is in the safe zone, 1cm lateral to the ventromedial region towards the root of the V nerve, approximately 1cm in size; this approach must be used to minimize deficits.17
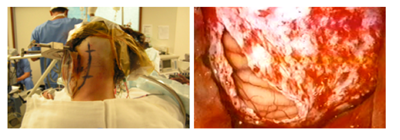
The classic retrosigmoid approach (Figure 1) presents the ventral part of the pons as a limitation; the extension of this approach is an alternative, with removal of a part of the mastoid bone and the temporal bone and a 50% increase of the angle of vision, which allows the ventral approach with lower cerebellar traction.22 Hauck EF et al.23 described 9 cases using pre sigmoid approach (Figure 2) with access to the lateral part of the pons, between the facial and trigeminal nerves, perpendicularly towards the brainstem.
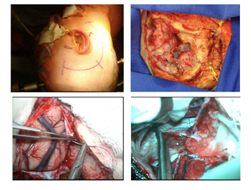
Sub temporal transtentorial approach is used for cavernomas located at the top of the pons; the access is performed through the middle cranial fossa with temporal lobe retraction, cut of the cerebellar tentorium, but only after the identification of the IV nerve.15
Anterior transpetrosal approach (Kawase approach) is indicated for lesions located in the inferior part of the pons with access through the middle fossa, extensive dissection, drilling of the petrous apex, and opening of the tentorium, visualizing the superior petrosal sinus.5,15 Peng Hu et al.17 used this approach in 9.6% of the cases and Alba et al used this approach in 5% of the cases.
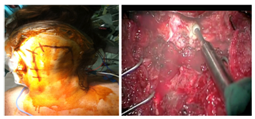
Far lateral approach (Figure 3) is indicated for lesions located in the lower clivus in the anterior part of the pons; as an advantage, it presents a smaller retraction of the cerebellum and brainstem. However, this approach requires a long surgery and can cause craniocervical instability and vertebral artery lesion.8 Trans oral transclival approach (Figure 4) is an alternative for these lesions located in the lower part of the pons.15
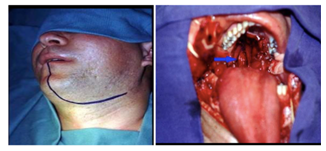
Endonasal transclival approach is indicated for cavernomas in the ventromedial region of the pons because it presents a direct access and manipulates less the cerebral tissue, which reduces postoperative morbidity and hospitalization time (Figure 5). The complication of this approach is the risk of cerebrospinal fluid leak, even though it can be minimized with lumbar drainage and small bone defects.1,3 The endoscopy requires a great surgical ability and adequate tools; the surgeon must be trained and the equipment must be available. Kassan et al.24 classified the complexity of endoscopic lesions from I to V; anterior pontine cavernomas have a classification V.
Postoperative rebleeding is associated with residual cavernoma; however, the presence of asymptomatic residual lesion does not justify a new surgical procedure.9,18
Radiosurgery is not the first-choice treatment because of a 14% to 40% risk of bleeding during the latency period of the treatment and its complications are directly related to the dose of radiation used.15 It is an alternative for lesions that are considered unresectable and observation must be chosen.2
Neuroendoscopy, neurophysiological monitoring, tractography, intraoperative ultrasound, and neuronavegation are extremely useful nowadays. The diffusion tensor imaging (DTI) tractography demonstrated the displacement of the corticospinal tract and can facilitate the surgical procedure.25 Neurophysiological monitoring during the procedure of dissection and excision of the lesion is extremely useful for the monitoring of corticospinal tract lesions(Table 2).
Upper pons |
Lower pons |
Endoscopic approach |
Endoscopic approach |
Subtemporal transtentorial approach |
Far lateral approach |
Presigmoid approach |
TransoralTransclival approach |
Retrosigmoid approach |
Retrosigmoid approach |
Table 2 Cavernomas approaches.
Anterior pontine cavernomas have a high risk of bleeding and an extremely high risk of rebleeding; therefore, they must be treated as soon as they are diagnosed due to the high risk of morbidity and mortality. The treatment is through surgery and the approach must be chosen depending on the topography in the upper and lower part of the pons and the ability of the neurosurgeon with the endoscope. Total resection and the cure of the lesion must always be aimed.
Nil.
None.
The author declares no conflicts of interest.

©2017 Neves, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.