Advances in
eISSN: 2572-8490


Review Article Volume 3 Issue 2
1Department of Materials Engineering, University of São Paulo, Brazil
2Department of Nanomedicine and Nanotoxicology, University of São Paulo, Brazil
Correspondence: Rafael Salomão, Materials Engineering Department, São Carlos School of Engineering, University of São Paulo, Avenida Trabalhador São-carlense 400, São Carlos, São Paulo, Brazil, Tel 55-1633739576, Fax 55-1633739590
Received: August 14, 2017 | Published: November 8, 2017
Citation: Costa LMM, Olyveira GM, Salomão. Precipitated calcium carbonate nano-microparticles: applications in drug delivery. Adv Tissue Eng Regen Med Open Access. 2017;3(2):336–340. DOI: 10.15406/atroa.2017.03.00059
Calcium carbonate (CaCO3) precipitated particles present huge applications in drug delivery systems because their characteristics (such as size distribution, crystalline phase and specific surface area) can be precisely tailored during the synthesis. Besides this, most of the processing methods present competitive costs and produce highly biocompatible materials. This work reviewed briefly some of the many chemical and biological synthesis methods for CaCO3 nano-microparticles as well as their combination with different macromolecules (active principles, polyelectrolytes and DNA).
Keywords: precipitated calcium carbonate, calcite, aragonite, vaterite, drug delivery, nano-microparticles
PSS, poly (sodium 4-styrenesulfonate); SDBS, sodium dodecyl benzene sulfonate; CDJP, controlled double-jet precipitation; BSP, betamethasone sodium phosphate; Gly, glycine; EG, ethylene glycol; PAH, poly (allylamine hydrochloride); PUA, poly (urethane-amine); CMC, carboxylmethyl cellulose; CMCh, carboxymethyl chitosan
Calcium carbonate (CaCO3) is an abundant mineral that has several uses in paper,1 Portland cement1 and steel making,3 as agricultural input for soil modification4 and food additive in soy milk and dairy products. Due to its high biocompatibility, CaCO3 is also employed in many pharmaceutical (anti-acid, dietary supplement for child and postmenopausal women), biological (scaffolds for cellular and bacterial growth) and biomedical (bioactive material for drug delivery, base for orthodontic cements) applications.5–9 Its particles can be found as three different polymorphs (vaterite, m-CaCO3, aragonite, λ-CaCO3, and calcite, β-CaCO3) in a wide variety of size, shape and crystalline structure of different properties and characteristics.
Calcite is of the most common minerals on Earth due to its thermodynamic stability. It is the main constituent of sedimentary limestone rocks and has a rhombohedra crystalline structure.10 Aragonite (also orthorhombic) occurs in the skeletal parts of calcareous organisms,11 and in the by-products of the some denitrifying bacteria ("Bacterium calcis", or “Pseudomonas calcis 6”12). Vaterite can be found in eggshells, salmon otoliths in freshwater, as component of some organism's endoskeleton and has been observed as a constituent of salts found in bile.11,13 Recent researchers found that vaterite is composed of a major phase of hexagonal symmetry filled with amorphous nanodomains.14 Differently from calcite and aragonite polymorphs that have faceted crystals, vaterite particles are usually spherical or irregular15 (Figure 1).
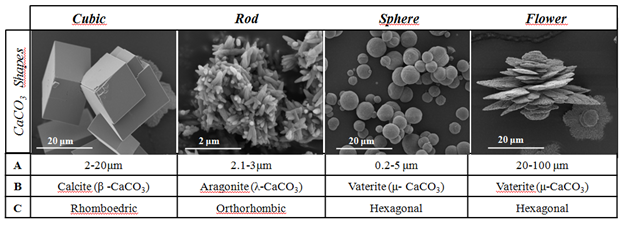
Figure 1 The typical shapes of calcium carbonate (CaCO3) particles.
*A: Average Diameter; B: Crystalline Phase; C: Crystalline System
Recently, vaterite particles were pointed out as having a huge potential for being use as drug delivery carriers and as bioactive material.16,17 Such potential arises from its lower thermodynamic stability, high solubility, and defect-rich microstructure that can accommodate many host-molecules and release it in a controlled way. Studies show that vaterite particles can be encapsulated or linked with various biological substances such as biopolymers (CMC, alginate), proteins, poly electrolytes and molecules such as DNA.18–21 Since the drug releasing parameters presented a strong relationship with particles’ characteristics (size distribution, specific surface area, internal porosity, and morphology) that are set during their synthesis, this work presents a brief review of the many methods employed to produce vaterite particles for biomedical application. We also collected useful information regarding the combinations of CaCO3 particle-active principle for drug delivery applications and their potential toxicity in some cases.
Methods for CaCO3 particles’ synthesis
There are several known synthesis methods for obtaining nanometer and micrometer materials that can be divided from top down to bottom up, where the larger scale can be manipulated to the desired scale and from the bottom up where the material is grown with atom control for atom (or molecule for molecule) respectively.22 The synthesis of calcium carbonate can be based on chemical.16,23,24 or microbiological.7,25–28 methods.
The most commonly used methods are those based on reactions in solution, leading to the formation of colloids in dispersed particles in a suitable solvent. Guo et al.29 showed the influence of several solvents on the crystalline phase of calcium carbonate. They demonstrated that using DMF as solvents there was produced calcite with polyhedral format. Mixing DMF (dimethylformamide) and methanol resulted in small rectangles with a small cavity in the center of each face. When DMF/n-propanol was employed, there were small beads of vaterite phase, and by adding DMF and ethanol the beads had larger diameters.
Chen et al.30 used a reaction of CaCl2+Na2CO3 at different temperatures and observed that at 25˚C there is calcite phase. It was obtained at 80˚C, the aragonite phase. For the formation of the vaterite phase, it was necessary to add an amino acid (glycine).31 Glycine is used as an additive to induce the crystallization of vaterite, because it is a less stable crystalline phase, the addition of chemical molecules is necessary for the synthesis of vaterite. Vaterite can be stabilized with various compounds, among them glycine (cited above),31 proteins,19 anionic surfactant (sodium dodecyl benzene sulfonate (SDBS)32 and poly (4-sodium styrene sulfonate) (PSS).24
In the double-jet method or CDJP (controlled double-jet precipitation) the cation and anion solutions are added simultaneously through separate lines to a stirred solution of a lyophilic polymer.33 This method was used by Jiang et al.34 in the first time for polymorphism study of CaCO3 in aqueous solution under mild conditions, without any organic additives. They showed that increasing temperature, there were different polymorphs for CaCO3 as can be seen in Figure 2.
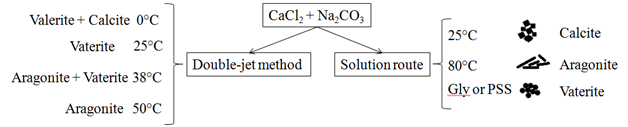
Figure 2 Differences between two methods (Double-jet and soluction route) for polymorphic calcium carbonate production using the same reagents [30,34,35].
Gly: Glycine; PSS: Poly (Styrene Sulfonate)
There is still a lot of divergence in the literature on the mechanism of calcium carbonate crystals growth, some researchers think that mechanism is spherulitic crystal growth and some assume the nano-agglomeration.36 The stabilization of the calcium carbonate crystals is one challenge related to these procedures, precisely to keep the particles stable in a certain size range, avoiding the agglomeration.37 Combining individual structures together to form large structures through sintering or Ostwald ripening, agglomeration is a way to reduce surface energy.
For the induction of CaCO3 precipitation from microorganisms, a solution and microbial cells with biochemical activities are required. These microorganisms are capable of secreting one or more metabolic products (carbonate ions) which react with the calcium ions present in the environment. The most accepted microbiological method uses bacteria in which they produce urease.38,39 Urease (urea amidohydrolase, EC 3.5.1.5) has the ability to induce the precipitation of carbonates in microorganisms. Some of the bacteria that produce high concentrations of urease can precipitate the calcite phase: B. thuringiensis40 Sporosarcinapasteurii;41 Bacillus sp. CR2.42
Certain bacteria also produce crystals of the aragonite structure. Bacteria is able of CaCO3 production in liquid medium, called "Bacterium calcis", or “Pseudomonas calcis 6”.12 The production of calcium carbonate crystals can occur in Pseudomonas cultures in an artificial seawater medium containing Na2CO3 or (NH4)2CO3. Researchers reported the same result with marine yeast, and claimed that calcium crystals resulted from accumulation of calcium deposits on the surface of cells in the aragonite structure.43,44 Rivadeneyra et al.26 presented an article in which they studied precipitation of calcium carbonate by Deleya hatophila using solids. It was studied in liquid media different concentrations of NaCl as the single salt and different incubation temperatures. All tested samples were able to precipitate calcium carbonate under different environmental conditions. The formed polymorphs crystals were calcite and vaterite. The relationship between calcite and vaterite depends on the total salt and on the type of medium. Subsequently, Rivadeneyra et al.45 showed that the formation of calcium Carbonate crystals in the aragonite phase using the same bacterium Deleya halophila is a sequential process that begins with a produced nucleus by the agglomeration of some calcified bacterial cells and the subsequent accumulation of more calcified cells and carbonate, which acts to join the bacteria together. The process leads to the formation of spherical aragonite with 50μm in diameter. This Mineral phase and its crystalline may be similar to those found in inorganic media.
Chen et al.27 synthesized vaterite by microbiological method with hollow bead of 10μm. When used Proteus mirabilis/Urea after 5 days of reaction at (27˚C), a vaterite phase was formed. However, when it was used Proteus mirabilis/CaCl2 solution with an ammonium carbonate diffusion method in 5 days of reaction at (27˚C), it was produced vaterite-calcite phases. In this way, it was to control phases by the choice of solution. Rodriguez-Navarro et al.25 and Chekroun et al.28 also studied calcite and vaterite formation by Myxococcus Xanthus bacteria. Rodriguez-Navarro et al.25 studied the changes in phosphate concentrations of culture medium leading to changes in local pH and Productivity. Besides, these factors change the structure and kind of CaCO3 polymorph precipitate (Vaterite or calcite). Chekroun et al.28 showed that calcite and vaterite produced in the presence of bacterium: Myxococcus xanthus, shows a range of morphologies that depends if the bacteria are alive or dead. They showed that both live and dead bacteria have a passive role on the precipitation of calcium carbonate, acting as a model for heterogeneity nucleation.
Drug delivery applications
Besides the knowledge of the structure and properties, surface modification, compatibility, and cell interaction for regenerative medicine, there is a need for targeting pharmaceutical drugs to local drug delivery for controlling diseases. The release rate can be controlled, depending on the size of the particles and their pores. Combinations of many carrier-drugs have been under study for the last three decades with the aim of solving the problems related to chemotherapy and radiotherapy. Conventional ways, being oral and parenteral administration of drugs, have several disadvantages due to the change of pharmacokinetic parameters and wide distribution throughout the body.
The adsorption of biomacromolecules inside porous calcium carbonate particles is presumably regulated by electrostatic interactions on the microparticle surface within pores and protein–protein interactions. Such assumption was presented by Sukhorukov et al.17 working with encapsulated porous calcium carbonate microparticles of an average size of 5 mm. Similarly, Wang et al.16 proposed a recent combination method for held drug release of adsorption on porous CaCO3 microparticles encapsulated with polyelectrolyte multilayer films formed by the LbL self-assembly. Ueno et al.5 described a easy method to load hydrophilic drugs and bioactive proteins into CaCO3 besides control the size of the particles. The drug release from the particles was confirmed by in vitro and in vivo experiments. Donatan et al.46 described a production of recent LbL templated based on spherical and nonspherical vaterite CaCO3 particles and analyzed geometry influences and polyelectrolyte multi layers on enzyme-catalyzed reactions.
Fujii et al.21 study hollow microcapsules of DNA and produced a assembling method using porous calcium carbonate microparticles as templates. So, DNA was adsorbed on surface calcium carbonate microparticles, and then DNA was covalently cross-linked on particles surface with ethylene glycol diglycidyl ether. Islan et al.47 developed hybrid biopolymer–CaCO3 microparticles treated with Alginate lyase enzyma, producing a mixed gel surface with a suitable size and a narrow distribution. Levofloxacin was used as a ideal drug to study the loading and delivery for application in pulmonary drug delivery.
Wei et al.48 study a simple method to produced hollow CaCO3 particles by the self-organization of nanocrystallites. Besides, one additional study using these particles as an anticancer drug delivery for Doxorubicin showed its advantages for local drug delivery detected by the pH value-sensitive structure, however, there is higher cytotoxicity due cellular uptake, perinuclear accumulation, and nuclear entry.
Zhao et al.20 produced polysaccharides and prepare microcapsules of chitosan and alginate as the multilayer-wall materials, and carboxylmethyl cellulose (CMC) as polyanions in the CaCO3 cores. Authors study the antitumor drug doxorubicin (DOX) and multilayer microcapsules for application in tumor treatment using in vitro and in vivo assays (Table 1).
CaCO3 Particles Synthesis |
Average Diameter |
Drug |
Ref. |
CaCl2/Na2CO3 |
5 μm |
Ibuprofen |
[16] |
Ca(NO3)2/Na2CO3 |
5 μm |
Doxorubicin |
[18] |
CaCl2/Na2CO3 |
140.7/ |
Betamethasone Phosphate |
[5] |
Ca(NO3)2 /Na2CO3 |
3-5 μm |
Doxorubicin |
[20] |
CaCl2/Na2CO3 |
4,6 μm |
Levofloxacin |
[47] |
CaCl2 and K2CO3 |
2μm |
Methylene Blue |
[49] |
CaCl2/Na2CO3 |
4 μm |
Doxorubicin |
[50] |
CaCl2/Na2CO3 |
5μm |
Doxorubicin |
[51] |
CaCl2/Na2CO3 |
4-6μm |
Ibuprofen |
[52] |
Ca(NO3)2 /Na2CO3 |
50-200nm |
Etoposide |
[53] |
Table 1 Synthesized differently CaCO3 particles for drug delivery applications.
PSS: Poly (Sodium 4-Styrenesulfonate); BSP: Betamethasone Sodium Phosphate; Gly: Glycine; EG: Ethylene Glycol; PAH: Poly (Allylamine Hydrochloride); PUA: Poly (Urethane-Amine); CMC: Carboxylmethyl Cellulose; CMCh: Carboxymethyl Chitosan
Calcium carbonate nano-microparticles can be produced by many methods, based on chemical or biological processes, resulting in a broad range of characteristics (size distribution, crystalline phase, morphology, specific surface area, internal porosity and solubility). Such particles found huge applications in drug delivery systems, both as main host unities of as microcapsules in combination with polymers and other macromolecules. Amongst CaCO3 polymorphs, vaterite particles seem to be the most interesting ones for this application due to its spherical shape, defect-rich structure and lower solubility. Depending on the drug adsorption-release conditions aimed, CaCO3 particles’ characteristics can be tailored during the chemical or biological synthesis process.
Authors acknowledge the Brazilian research foundations FAPESP (2010/19274-5), CNPq (308762/2014-2) and CAPES (PNPD20131739-33002045017P6) for supporting this work.
The author declares no conflict of interest.

©2017 Costa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.