Advances in
eISSN: 2572-8490


Research Article Volume 3 Issue 1
1Department of Chemistry, Shanmuga Industries arts and Science College, India
2PG and Research Department of Chemistry, KMG College of Arts and Science, India
3PG and Research Department of Chemistry, Muthurangam Govt Arts College (Autonomous), India
Correspondence: Kannappan Geetha, PG and Research Department of Chemistry, Muthurangam Govt Arts College (Autonomous), Vellore, 632 002, Tamil Nadu, India, Tel 91 09486925596, 91 0416 2262068, Fax 91 0416 2263768
Received: August 18, 2017 | Published: November 6, 2017
Citation: Karthik AD, Shakila D, Geetha K . Eco friendly synthesis, spectroscopic investigation of copper and its oxide nanoparticles with excellent electrical conductivity. Adv Tissue Eng Regen Med Open Access. 2017;3(1):324–328. DOI: 10.15406/atroa.2017.03.00057
A facile and rapid route for the synthesis of uniform Nano copper and its oxide (CuO NPs) by a microwave assisted method is demonstrated. The copper and its oxide nanoparticles (CuO NPs) prepared exhibit excellent conductivity, which is comparable with electrospun copper nanofibers. Among the various metal nanoparticles, Copper and its oxide nanoparticles (CuONPs) have attracted considerable attention because copper is one of the most important in modern technology and is readily available. Copper Nanoparticles have been much attractive because it is easily available, cost effective and conducting nature. Copper and its oxide Nanoparticles were synthesized using Copper (II) succinate by microwave conventional method. Copper and its oxide Nanoparticles so obtained were characterized by UV- Visible spectroscopy, Fourier Transform Infrared Spectroscopy and X-Ray diffraction analysis. X-Ray diffraction analysis proved the formation of Copper and its oxide Nanoparticles. SEM and AFM analyses showed the presence of Nanoparticles.
Keywords: copper sulfate, uv visible, xrd, ft-ir, AFM
Metal Nanoparticles have attracted considerable attention because of their excellent electronic and optical properties. They have been used in many fields, such as displays, solar cells, touch-screens, sensors, and electromagnetic interference shielding.1 However, for most of these applications, metal Nanoparticles with high electrical conductance are required. Compared with various other metals, copper is more abundant and less expensive, while having favourable mechanical, electrical, and optical properties.2 Copper Nanoparticles have thus been suggested as a low cost alternative to indium tin oxide (ITO), investigated extensively and compared against conductive polymers, carbon nanotubes, graphenes and Ag nanowires.3–6 To date, various methods have been developed for synthesizing Copper and its oxide Nanoparticles (CuO NPs), including electrochemical deposition, electrospinning, template processes, hydrothermal reduction and reverse micellar systems.7–12 On the other hand, the microwave-assisted synthesis.13,14 which is generally quite fast, simple and efficient in energy, has been developed and is widely used in various fields such as molecular sieve preparation, radiopharmaceuticals, the preparation of inorganic complexes and oxide, organic reactions, plasma chemistry, analytical chemistry and catalysis. Generally speaking, the power, heating frequency and on/off irradiation cycles are the main heating parameters of a microwave oven and each of them may have a great effect on the structure and properties of the products. To the best of our knowledge, although such a heating method has been a focus of research, most of previous reports were limited to fixed working conditions of the microwave oven.15–20 and there is no comprehensive report addressing the effects of these heating parameters on the synthesis of nanomaterials.21–23 Furthermore, there are few reports on synthesizing the Copper and its oxide Nanoparticles without template at mild condition by the use of microwave method. Here, we report a simple microwave method to fabricate well aligned Copper and its oxide Nanoparticles (CuO NPs) on a Copper surface without any template and surfactant.24–26
Materials
All the chemicals, reagents used in our experiments were of analytical grade and were used as received without further purification. Succinic acid, Copper sulphate and NaOH were purchased from Aldrich and were used as received, hexane SDF and ethanol from distillery.
Synthesis of the Copper(II) succinate precursor
The CuSO4.5H2O (2mmol) was dissolved in 10 mL of distilled water to form a homogeneous solution. A stoichiometric amount of sodium hydroxide (NaOH) and succinic acid were dissolved in distilled water. The sodium succinate thus formed was drop wise added into the above solution under magnetic stirring. The solution was stirred for about 30min and a green precipitate was obtained which was centrifuged and washed with ethanol several times. The product was dried. The Copper (II) succinate was characterized various spectral techniques.27
Synthesis of the copper and its oxide nanoparticles
Copper and its oxide nanoparticles were synthesized by a domestic microwave method in the presence of Copper (II) succinate. The Copper (II) succinate placed in a domestic microwave. Finally, the reaction system was heat treated at 473K for 2h. Copper and its oxide Nanoparticles were obtained black powder (Scheme 1 & 2).
UV Visible Spectra
Copper Nanoparticles typically exhibit~500nm. However the Copper (II) succinate show an absorption peak at 264,372 and 568nm (Figure 1) and also Copper and its oxide Nanoparticles synthesized here show an absorption peak at450 nm which can be assigned to the absorption of Nanoparticles of copper. Tauc plot for synthesized Nanoparticles is shown in Figure 1. This peak can be assigned to the absorption of Nanoparticles of copper. A modest blue shift of the absorption edge relative to that of the bulk copper powder is observed. This observation alludes to the size effect of copper and its oxide Nanoparticles.
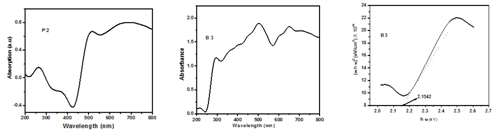
Figure 1 UV-Visible spectrum of Precursor P2, Copper and its oxide Nanoparticles of B3 & Tauc plot of B3.
FT - IR measurement
FT-IR spectroscopy is a useful tool to understand the functional group of any organic molecule Figure 2A shows IR spectrum of Copper (II) Succinate precursor. Figure 2B shows IR spectrum of copper Nanoparticles. A twin peak at 621cm-1 & 680cm-1 indicated the Cu-O Stretching vibration. The metal salt (Cu-O-C) Peak appeared at 1192cm-1. A Peak at 3541cm-1 indicates OH stretching of the water in the precursor which disappeared in the Nanoparticles. A Peak at 1637 cm-1 indicates metal carbonyl (C=O) of the precursor which disappeared in the Nanoparticles B3.28–30
X-Ray diffraction studies
XRD pattern of Copper and its oxide nanoparticles deposited at 300˚C under nitrogen environment with a collecting angle varying from 20 to 80˚ and the usual copper Kα1 radiation λ=1.5406Å is shown in Figure 3 & Table 1. The hkl values (111), (200), (220), 2θ values 48.96, 53.67, and 68.24 and corresponding d values, 2.073, 1.796, and 1.274Å are used for determining lattice parameter which came to be 3.01, 4.01, and 8.00Å, respectively using standard formula (Lattice=d√h.k.l). The relative peak intensities and the position of diffraction peaks in the XRD pattern are very similar to those reported in the JCPDS standard data 40836 and others. The Table 2 shows particle size of Copper and its oxide nanoparticles. The position and the integral width of x-ray diffraction peaks are determined to identify the crystalline phase which is found to be face centered cubic.31
|
2θ |
d |
1000/d2 |
(1000/d2) / 77.32 |
hkl |
|
48.96 |
2.073 |
232.07 |
3.01 |
111 |
|
53.67 |
1.796 |
310.02 |
4.01 |
200 |
|
68.24 |
1.274 |
616.11 |
8 |
220 |
Table 1 Peak indexing from d-spacing.
|
2θ of the Intense Peak (deg) |
θ of the Intense Peak (deg) |
FWHM of Intense Peak (β) Radians |
Size of the Particle (D) nm |
d Spacing nm |
Plane |
Material |
Microstrain |
|
32.68 |
16.34 |
0.1378 |
60.4 |
2.7401 |
(202) |
CuO |
0.1175 |
|
35.75 |
17.875 |
0.1574 |
53.3 |
2.5115 |
(110) |
Cu2O |
0.122 |
|
38.98 |
19.49 |
0.2362 |
35.7 |
2.31055 |
(111) |
Cu |
0.1668 |
|
48.96 |
24.48 |
0.1968 |
44.5 |
1.8604 |
(200) |
Cu |
0.108 |
|
53.67 |
26.835 |
0.1378 |
65 |
1.7077 |
(20) |
CuO |
0.0681 |
|
58.49 |
39.245 |
0.2166 |
42.1 |
1.578 |
(200) |
Cu |
0.0967 |
|
61.72 |
30.86 |
0.1378 |
67.5 |
1.5029 |
(200) |
Cu |
0.576 |
|
66.44 |
33.22 |
0.1368 |
48.4 |
1.4072 |
(200) |
Cu |
0.0751 |
|
68.24 |
34.12 |
0.168 |
57.3 |
1.3743 |
(20) |
CuO |
0.06198 |
Table 2 The particle size of B3.
Particle size calculation: The existence of sharp peaks indicates that the synthesized nanoparticles are polycrystalline in nature.31 One can calculate the values of average crystallite size Debye - Scherer formula (D) from eq (1), d-Spacing the value of d (the inter planar spacing between the atoms) is calculated from equation (2) and microstrain (ε) from equation (3) formation XRD spectrum using the following equations:
(1)
Bragg’s Law: (2)
(n=1)
Wavelength Å for Cu Ka
(3)
The calculated d-spacing and micro strain details are in Table 2.
Cyclic voltametry
Figure 4 shows cyclic voltammogram (CV) the instructor will familiarize the students with the operation of the computer program interfaced to the CV and the proper procedure for introducing and removing solutions from the cell. Set the Copper Nanoparticles was recorded in DMSO with 0.1M tetrabutyl ammonium perchlorate as supporting electrolyte in the initial potential +2V and –2V. The scan should be initiated in a positive direction with a scan rate of 50mV/s with a conventional three electrode system composed of a platinum auxiliary, Glassy carbon working electrode and Calomel (Saturated KCl) reference electrode. The reductive peaks correspond to Cu (II)/Cu (I) and Cu (0) i.e CuOàCu2OàCu. After the deoxygenating is complete switch on the working electrode and wait for approximately 10 s until the current has reached a constant value. Initiate the potential scan and a background CV of the supporting electrolyte is obtained. This scan with a wide potential range provides the operational limits of potential at which the experiment can be run, i.e. the potential window of the electrolyte. The redox potential values obtained from the cyclic voltammogram for the Copper and its oxide Nanoparticles were quasi-reversible and the electron flow was sluggish (Table 3). Data from Cyclic voltammetric measurements; E1/2 is calculated as average of anodic (Epa) and cathodic (Epc) peak potentials E1/2=1/2(Epa+Epc)
SEM & AFM analysis for copper and its oxide nanoparticles
|
Compound |
Epc (V) |
Epa (V) |
∆E(V) |
E 1/2 |
ipc (µA) |
ipa (µA) |
ipa/ipc |
|
P2 |
-1.0901 |
0.9035 |
1.9936 |
0.0933 |
1.6120X10-4 |
7.782X10-5 |
0.4827 |
|
B3 |
-0.8354 |
-0.5376 |
0.2978 |
-0.6865 |
7.993X10-6 |
2.162X10-6 |
0.2704 |
Table 3 Redox Potentials of Copper and its oxide nanoparticles in DMSO solution at 298K.
The morphology of the product is examined by SEM. Figure 5 depicts the SEM pictures of a sample of Copper and its oxide Nanoparticles. From the micrograph, it was observed that the Nanoparticles are agglomerated. The Atomic force microscopic (AFM) photographs of the product are given in Figure 6. These sizes are nearly cubic (fcc). The area roughness and RMS are in the range 160 to 487nm which indicates its conducting nature. We may consider that when the reaction was carried out at 473K, most organic molecules decomposed. Copper and its oxide nano have rod like, loose solid morphologies.
In summary, a facile and rapid route has been developed for synthesizing uniform crystal Copper and its oxide nanoparticles by a microwave assisted method. This method is highly efficient and is scalable for large scale synthesis. The prepared Copper and its oxide nanoparticles have an average diameter of 50nm, 10μ and lengths of longer than 10mm, i.e., an aspect ratio of greater than 300. Moreover, the Copper and its oxide nanoparticles show outstanding conductivity, comparable with electrospun copper nanofibers. Our experimental results demonstrate that this novel and simple route can produce high quality crystalline Copper and its oxide Nanoparticles of nanostructures.
Authors are grateful thank to Principal, Muthurangam Govt. Arts College (Autonomous), Vellore, for providing facilities to undertake this work. We also thank Center for nanotechnology, VIT Vellore for AFM analysis.
The author declares no conflict of interest.

©2017 Karthik, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.