Advances in
eISSN: 2572-8490


Research Article Volume 3 Issue 1
Department of Applied Biology, Sree Chitra Tirunal Institute for Medical Sciences and Technology, India
Correspondence: Anil Kumar PR, Division of Tissue Culture, Department of Applied Biology, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, 695 012, India, Tel 91-4712520311, Fax 91-471341814
Received: June 19, 2017 | Published: October 26, 2017
Citation: Madathil BK, Kumary TV, Kumar AP. Self organized skin equivalent by epithelial and fibroblast cells on polycaprolactone electrospun mat with porous fibers. Adv Tissue Eng Regen Med Open Access. 2017;3(1):317–322. DOI: 10.15406/atroa.2017.03.00056
Tissue engineered skin substitutes are used in chronic, partial or full thickness wounds, and serve to augment the growth and repair of the skin at the wound site, by acting as a temporary or permanent skin replacement. In this study Polycaprolactone a biodegradable polymer was electrospun in a binary solvent system to form nonwoven mats with porous fibers (PPCL). These mats were hydrolysed with alkali to make them more hydrophilic and promote cell adhesion. The electrospun mat served as a scaffold for culture of human keratinocyte (HaCaT) and rabbit hair follicle dermal fibroblast cells (HFDF) cells. F-actin staining and SEM analysis showed that the cells exhibited their characteristic morphology, with good adhesion and spreading. Live dead staining confirmed the viability of the cells on the PPCL mat. A bioengineered construct of HaCaT and HFDF cells cultured on either side of the PPCL mat was generated in vitro. Light microscopy of Toluidine blue stained cryo sections of the construct reveal that the HaCaT cells formed intact multilayers, while the fibroblasts showed a more distributed growth pattern with migration into the mat, hence emulating the epidermis and dermis of the skin. The results were substantiated in the SEM micrographs and fluorescent images of DiI and DiO labeled cocultures. The PPCL mat cocultured with HaCaT and HFDF cells thus finds potential as a self organized tissue engineered skin substitute.
Keywords: polycaprolactone, skin equivalent, tissue engineering, electrospinning
The skin is the largest organ of the body. As the skin forms the outermost covering of the body, it is susceptible to trauma and injury from extrinsic factors. The loss of skin integrity with consequent loss of its function leads to a wound. The success of recovery of a wound depends on the regime of wound management adopted. The use of a proper wound dressing is a pivotal part of wound management. Wounds may be either acute or chronic. Further based on depth, wounds may be classified as superficial, partial thickness, full thickness and deep wounds. Selection of the type of wound dressing depends on the extent of trauma, amount of exudates and the overall wound environment. An ideal wound dressing should be non-toxic, non- allergen, facilitate wound healing, removal of exudates from the wound bed and act as a barrier to microbes.1 Wound dressings are either synthetic as in Tegaderm®, Opsite®, Biobrane™ etc or biological in origin as in autografts, allografts, xenografts, human amnion, oasis wound matrix® etc. Alternatively, newer strategies in science has led to the development of tissue engineered skin substitutes. These products are designed to aid wound closure and promote healing and in the long run serve as either a temporary skin replacement that helps to augment the growth and function of adjacent host tissue or to serve as a permanent skin replacement to replace the full thickness of the skin layer and improve the quality of the skin at the wound site. TransCyte™, Epicel®, Integra™, Apligraft® etc are some of the well known bioengineered skin substitutes in the markets.2
Some of these commercialized products are biosynthetic in that they comprise of both a polymer component, the biomaterial as well as a biological component ie., the seeded epidermal keratinocyte and/or the neonatal or dermal fibroblast cells. The polymers commonly used are type 1 bovine collagen, chondroitin-6-sulphate, hyluronan, PLGA (poly lactic-co-glycolic acid and polygalectin).3 Electrospinning is an advanced technique by which polymers in solution are used to generate non woven micro and nano fibrous mats. The use of electrospun mats for cell cultivation, wound dressings and drug delivery have been reported earlier.4–6 The mesh structure of the mat permits nutrient infiltration exchange of air and waste exudation. Polycaprolactone (PCL) is a biodegradable polymer and is used for electrospinning due to its spinnability and strength. Electrospun PCL is widely used for tissue regeneration and wound healing application due to its high biocompatibility and the similarity of its nanofibers to collagen fibrils of natural extracellular matrix.7 PCL mats loaded with drugs or bioactive components have also been proved to augment wound healing.8
Full-thickness skin substitutes are composed of both epidermal and dermal layers. Either autogenic or allogenic cells are used to generate the bioengineered construct. Keratinocytes form the upper layer like the epidermis while fibroblasts are used to form the inner layer that is analogous to the dermis. In the current study, polycaprolactone was electrospun using a dual solvent system to form non woven mats with porous fibers. The scaffold was surface treated and seeded with keratinocyte cells and hair follicle dermal papillae fibroblast cells on either side of the mat to generate a tissue construct which can be used as a potential skin substitute for partial and full thickness wounds.
Fabrication of electrospun mats
Electrospun PCL mats having pores (PPCL) was fabricated as previously reported.9 A 10% PCL (average Mn 80000, Sigma) solution was prepared using a binary solvent system of Terahydrofuran and Dimethyl sulphoxide. The polymer was taken in a syringe with a blunt end needle and connected to a customized electrospinning setup (Holmarc Opto-Mechatronics Pvt Ltd, India). A grounded plate placed at distance of 12 cm a tip of the needle was used as a collector. Electrospinning was performed at a potential difference of 12kV with a flow rate of 1.5ml/h. The mat from the collector was detached, rinsed with Milli-Q water and dried at 37 °C inside a hot air oven. PPCL mats were cut into 2×2cm size and treated with 0.1M NaOH for 15min at room temperature for surface hydrolysis. The mats were then rinsed in deionized water, sterilized with 70% ethanol. The mats were transferred into a biosafety cabinet (Esco), further rinsed with sterile deionized water and air dried.
Cell culture
Human Keratinocytes (HaCaT, NCCS Pune, India) were maintained in Dulbecco’s Minimum Essential Medium: F-12 Hams (DMEM:F12, Gibco) supplemented with 10% Foetal Bovine Serum (Gibco), and Penicillin-Streptomycin antibiotic (Pen Strep-10,000 U/ml) (Gibco).
Hair follicle dermal papillae fibroblast cells (HFDF) were isolated from rabbit vibrissae (New Zealand White Rabbits). Briefly, the upper lip was shaved off and the fad pads containing the vibrissae was dissected out and collected in Phosphate Buffered Solution (PBS) containing Antibiotic antimycotic solution (AAS) (Gibco) and Pen Strep-10,000U/ml. The tissue was rinsed thoroughly with 10% betadine solution in PBS followed by multiple washes in PBS containing the AAS and Pen Strep. The skin was peeled off and remaining tissue with hair follicle was allowed to be digested using protease (Streptomyces griseus: 2mg/ml, P8811, Sigma, USA) for 3hrs at 37˚C. The hair follicle with the bulb was pulled out using a pointed fine tip forceps, washed with PBS, and transferred to Collagen (Type IV; C-5533, Sigma) coated tissue culture dishes (35mm; Nunc). The culture medium used to initiate and maintain the explant culture was Dulbecco’s Minimum Essential Medium: F-12 Hams (DMEM: F12, Gibco) supplemented with 10% Foetal Bovine Serum (Gibco), and Penicillin-Streptomycin antibiotic (Pen Strep-10,000 U/ml) (Gibco). The explants were incubated in a CO2 incubator (Sanyo, Japan) set at 37 °C and 5% CO2 with>90% relative humidity. The HaCaT and HFDF cells were maintained with medium change on every third day. Cells were passaged by normal trypsinization (0.25% Trypsin- 0.02% EDTA; Gibco) and seeded at a split ratio of 1:2.
Cell adhesion on PPCL mats
The PPCL mats were preconditioned with culture medium for 10minutes inside the biosafety cabinet prior to cell seeding. Around 1×06HaCaT and HFDFC were seeded on respective PPCL mats (2×2cm). The cell seeded mats were maintained for five days with medium change every 48h.
Viability and Morphology of the cells on PPCL: The viability of the adhered cells was determined after 48h by live-dead staining. The live-dead stain was prepared by mixing 5µg/mL of FDA (Sigma, India) in serum-free medium and propidium iodide (0.05µg/mL). The cells were incubated with the stain for 10min, rinsed with PBS and viewed under a fluorescence microscope (Leica DMI 6000, Germany) using I3 (green) and N21 (red) filters.
The morphology of the adhered cells after five days culture was evaluated by staining of the cytoskeletal actin filaments. Briefly the cells were fixed with 4% paraformaldehyde (Sigma). The cells were washed twice in PBS and permeabilized using 0.1% Triton X-100 (Sigma) for 5min. Cells were rinsed again with PBS, incubated for 20min with Rhodamine-Phalloidin (1:100;Sigma) and visualized using a fluorescence microscope.
SEM analysis: The morphology of the adhered cells was also evaluated by scanning electron microscopy (SEM). The cultures were rinsed in Sorensen’s phosphate buffer and fixed in 3% Gluteraldehyde (Sigma). The sample was dehydrated in increasing concentrations of methanol (30%, 50%, 70%, 90% and 100%) and gold coated. The samples were viewed under a scanning electron microscope (FEI Quanta 200 Environmental Scanning Electron Microscope and FE-SEM, Hitachi 6600) and digital images taken.
Co-culture of cells on PPCL mat
A co-culture with keratinocyte and fibroblast cells on either side of the PPCL mat was established. HaCaT cells were seeded at high density (1×106cells) onto PPCL mat (2×2cm), and cultures were maintained in vitro for 3 days. A Teflon-O ring was placed inside a fresh culture dish and the center of the ring was filled with DMEM: F12 medium. The PPCL mat with the HaCaT cells was placed inverted, cell side down, onto the teflon ring such that the cells were in contact with the medium placed in the center of the teflon ring. Equal numbers of HFDF cells were seeded onto the other side of the PPCL mat. Fibroblast cells were allowed to adhere and spread and the culture medium was topped up as required. The cultures were maintained for another 5 days under standard culture conditions with medium change on every 48 h.
Morphological evaluation of the coculture: The PPCL mat with the dual culture was rinsed with sorenson’s phosphate buffer and fixed in 3% gluteraldehyde. The samples were processed and viewed under a scanning electron microscope as described above.
Evaluation of organized skin equivalent: The samples were fixed overnight in 10% neutral buffered formalin. A central portion of the mat was excised and embedded in cryoembedding medium (Leica Jung). Sections were taken at 7µm thickness using a cryostat set at -20 °C (Leica, CM3050S). The cross sections of the cell seeded PPCL mats were collected on poly L-Lysine (Sigma) coated glass slides and stained with toluidine blue. The stained sections were air dried, mounted (Leica Mounting medium) and cover slipped. The sections were viewed under a microscope (Nikon TS100) and images were recorded.
The epithelialization and fibroblast infiltration on PPCL mat was analyzed by tagging cells with different colors. HaCaT and the HFDF cells were labeled with DiI and DiO respectively (Vibrant cell labeling solutions, V22889, Invitrogen, USA) as per the manufacturer’s instructions. Briefly, the cells grown to confluency in a T-25 flask (Nunc) was trypsinised and resuspended in 1 ml of serum free medium. 5µl of the dye was added to the cells and incubated at 37°C for 15minutes. The cells were centrifuged and resuspended in serum containing culture medium and seeded onto the PPCL mats. Labeled HaCaT cells were allowed to establish a culture on the PPCL mat prior to seeding with labeled HFDF cells on the other side. Following in vitro culture, the sample were fixed in 4% paraformaldehyde (Sigma) for 2h and cryosections were taken onto poly-L-lysine coated slides as previously described. The sections were directly viewed under a fluorescence microscope with (red) N21 and (green) I3 filters (Leica DMI 6000B, Germany).
The percentage of infiltration of HaCaT and HFDF cells into the PPCL mat from the surface of seeding was calculated using the image analysis (Image J Software). Multiple images from different samples (n=3) were analyzed and the percentage of infiltration was calculated.
Fabrication and characterization of electrospun mats
Electrospun scaffolds could provide a microenvironment mimicking the ECM of tissues due to its size and morphological architecture.10 The fiber density and orientation greatly determines the cell infiltration into the mat. Generally, cells form a superficial layer on the surface of the scaffold by conventional seeding methods and remains a limitation in development of tissue engineered constructs. Tightly organized fibers perform similar to a flat surface and the cells tend to grow in two dimension rather than infiltrate to form a three dimensional growth pattern. Jinglei Wu et al.11 have reviewed various techniques to improve cell infiltration for electrospun scaffolds. Huizhi Chen et al.12 used a modified collector for improving cell infiltration by collecting fibers on water. Those techniques influence the bulk property of the electrospun mat. When two or more cell types are used the scaffold should support the growth characteristic required for each cell type.
In this study, we fabricated electrospun mat with porous fibers in order to address the growth requirements of skin epithelial and dermal fibroblast cells.9 A dual solvent system creates porous fibers of PCL by thermally induced phase separation.13 Scanning electron microscopy images showed the surface morphology of PCL mat with porous fibers (PPCL) (Figure 1A). The cell adhesion on hydrophobic PCL was improved by surface hydrolysis using alkali. Surface treatment did not alter the morphology or porosity of the fibers (Figure 1B). The fibers were porous, smooth, continuous and showed good connectivity between interfiber pores.
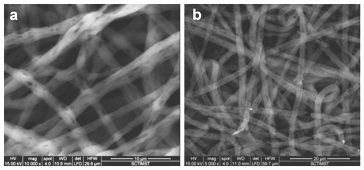
Figure 1 (a) SEM image showing pores on the PCL fibers. (b) Morphology of the fibers after surface modification with alkali.
Cell culture
Living skin substitutes require scaffolds with unique properties such as similarity to native ECM, selective cell adhesion and migration by different cell types. The epidermal component should restrict the growth of cells on the surface where as the dermal component like fibroblast should be able to promote cell proliferation and migration. It has been also reported that increased surface hydrophilicity and water uptake can decrease the fibroblast adhesion on polymers.14 However, strong cell adhesions between epithelial keratinocytes are not affected by the hydrophilicity and water uptake. Hence in this study, the electrospun mats were prepared with porous fibers and surface hydrolysed to improve fibroblast adhesion and migration at the same time restricting the epithelial cells on the surface. Various coculture systems have been proposed as a model in vitro system for skin tissue engineering. Gazzarri et al.15 adopted a coculture model of BALB/3T3 mouse embryo fibroblast and human keratinocyte (HaCaT) cell lines on three-arm-branched star poly (ε-caprolactone). Ashraf et al.16 used a coculture of HaCaT and primary human fibroblast cells to create bilayered nanofibers using combinations of polyvinyl alcohol and polyhydroxybutyrate. A similar approach by Luo et al.17 described about a bilayer of chitosan/PCL and PLLA mat, each side seeded with two different cell types. The bilayer with varied porosity, prevented intermingling of keratinocytes and fibroblasts.
Cell adhesion on PPCL mats: The electrospun mat fabricated in this study exhibits a homogenous fiber distribution and also has the fiber surface modified with pores and is hydrolyzed. HaCaT cells and primary HFDF cells from rabbit vibrissae adhered on the fibers of the PPCL mats and proliferated over five days in culture.
Viability and Morphology of cells on PPCL: HaCaT and HFDF cells were transferred on to PPCL mats by conventional top seeding method. The viability assessed by FDA/PI staining confirmed that the cells were viable on the PPCL mats (Figure 2A & 2B).
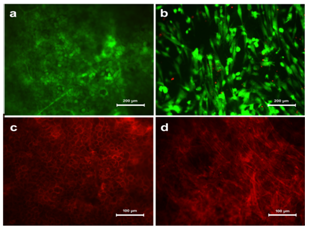
Figure 2 Viability of (a) HaCaT and (b) HFDF cells on PPCL as confirmed by FDA/PI staining. The viable cells appeared green while nucleus of non viable cells were appeared red. Morphology of adhered cells on PPCL mat visualized by actin staining. (c) HaCaT cells formed a sheet over the mat and (d) HFDF formed a distributed cell pattern.
Cells on the PPCL mats were cultured for five days and analyzed for cell adhesion. Actin cytoskeletal structures stained after five days in culture showed that both cells could adhere on the mat and exhibit their characteristic morphology. The HaCaT cells expressed specific epithelial like morphology whereas fibroblasts appeared in spindle shape (Figure 2C & 2D). The actin staining also showed overall distribution of cells on the surface of the PPCL mat. Evaluation of PCL based electrospun mats using HaCaT cells have been previously reported and the current study also showed good cell adhesion properties on the PPCL mats.18 HaCaT was tightly packed with intact cell-cell contact and HFDF seemed to be distributed with spindle morphology. This clearly confirms the same seeding method resulted in different cell distribution outcome suitable for skin substitute.
SEM analysis
The surface morphology of adhered cells were further analyzed by SEM. The results confirmed the observations of actin staining. Under high magnification, the HaCaT cells were found to form a continuous sheet over the surface of the PPCL mat (Figure 3A). This indicates that the surface modified PPCL served as a suitable substrate for the keratinocytes to adhere and form an epithelium. The cell coverage shown by HaCaT was similar to the previous report by Jun et al under air exposure conditions.19 The fibroblast on the other hand was distributed all along the surface of the PPCL mat exhibiting characteristic fibroblast spindle morphology (Figure 3B). The fibroblast spread over the fibers by extending the filopodia over and across the non woven mat. An in vitro study by Farrugia et al.20 showed that fibroblasts can bridge between fibers at a distance ranging from 8-133um. Over five days in culture, the fibroblasts adhered and spread indicating cell migration into the electrospun mat.
Co-culture of cells on the PPCL mat
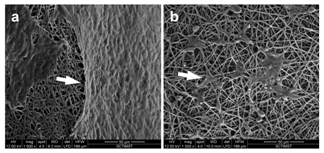
Figure 3 SEM images showing cell adhesion on the PPCL mats. Arrow designates the adhered cells. (a) Keratinocytes formed tight epithelial cell sheet over the electrospun mat. (b) Fibroblast cells spread over the fibers indicating cell infiltration.
Evaluation of electrospun mats for skin tissue engineering using coculture of cells belonging to different species is not new.21,22 It has also been shown that HaCaT cells can organize into intact epithelia in the presence of human or mouse fibroblasts.23 The cells selected for developing organotypic skin equivalent using PPCL was human keratinocytes and rabbit hair follicle fibroblast cells. To the authors knowledge this is the first report on an organotypic system using HaCaT and rabbit hair follicle fibroblast cells. This mix species coculture system is suggested as an organotypic skin equivalent and as an in vitro tissue model for evaluating biomaterials for skin tissue engineering.
Morphological evaluation of coculture on PPCL: The skin is comprised of an outer epidermis and an inner dermis. The skin equivalent model was achieved by coculturing HaCaT and HFDF cells on either side of the PPCL mat. The keratinocytes and fibroblasts on the PPCL mat were observed under SEM to evaluate the details of cell and material interaction. A well formed epithelial layer was evident on the mat upwards where as the fibroblasts formed isolated cell migration on fibers underneath (Figure 4). A similar culture method using human keratinocytes and mouse 3T3 fibroblasts on collagen-PCL biocomposite membranes formed bi-layered skin substitute.24 Here PPCL mats modified with surface treatment without adding any biomolecules showed reorganization of cells into a skin equivalent.
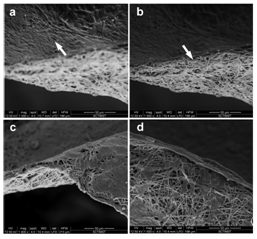
Figure 4 Morphological evaluation of coculture on the PPCL mats. Arrows indicates the cells adhered on the fibers. On one side of the mat, HaCaT formed a smooth monolayer (a), while the fibroblast got distributed on the other side (b). A closer observation showed the characteristic morphology of keratinocytes (c) and fibroblasts (d).
Evaluation of organized skin equivalent: Keratinocytes can form only a layer of epidermis that eventually undergoes apoptosis within 14 days in culture. However, when cocultured with mesenchymal cells, keratinocytes can proliferate and form definite multilayers.25 The simplest form of epidermal-dermal coculture is to culture keratinocytes on post mitotic dermal fibroblast feeder. Advanced 3D culture systems can provide proper stratified epithelia with intact dermis to mimic histological architecture of skin.22 In this study, a coculture cell culture system is described that contains epidermal keratinocytes and dermal fibroblasts.
The organization of keratinocytes and fibroblasts into a skin equivalent was analyzed by toluidine blue staining on cryosectioned coculture system. The sections showed very clear demarcation of epithelial and dermal (PPCL fiber) components. The upper side of the cross section showed fully covered lined epithelial cells evidenced by dark blue staining. The PPCL mat below the epithelial lining was densely stained confirming the presence of distributed fibroblast cells (Figure 5A).
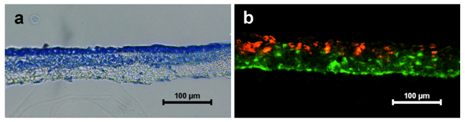
Figure 5 Self organization of skin equivalent when HaCaT and HFDF cells were cultured on either side of the PPCL mats. (a) Toluidine blue staining of coculture showing formation of upper epithelial layer and organization of lower dermal component. (b) Dual staining of HaCaT (red) and HFDFC (green) showing infiltration of fibroblasts into the PPCL mat.
Fibroblast infiltration is required to engineer a cell laden dermal equivalent. The porosity is critical in determining the cell migration inside the scaffold. Adjusting the inter-fiber distance or introduction of micropores into the electrospun mat are examples of improving cell infiltration.26 The inter fiber pore size and the distribution of the pores can be easily achieved by sacrificial components or adjusting the electrospinning conditions.27 The HFDF cells seeded on the PPCL mat also showed similar infiltration into the inter fiber space with even distribution of cells.
The fibroblast infiltration into the PPCL mat was confirmed by dual staining method. HaCaT cells tagged with DiI (red color) and HFDFCs labeled with DiO (green color) seeded on opposite sides of the PPCL mat confirmed self-organization of the cells into a skin equivalent. The cross section of coculture showed multilayered epithelial cells on upper side. The fibroblasts populated within the electrospun mat and expressed green color (Figure 5B). Image analysis of the fluorescent images revealed that HaCaT cells forming a sheet on one side infiltrated only upto 38.88±6.8 % of the thickness of the PPCL mat whereas HFDF cell migration was 89.57±6.3% (Figure 6). These results substantiate the formation of an organized epithelial layer on the top of the mat and more dispersed fibroblasts within the PPCL mat.
PCL is a slow degrading biomaterial in vivo due to its hydrophobic nature. Biodegradation of PCL is by hydrolysis rather than enzymes in the body. Random hydrolytic chain scission of the ester linkages reduces molecular weight and accelerates the degradation process. Various methods adopted to shorten the degradation time are by using low molecular weight polymer, co-polymerization with other lactones and alteration in surface morphology.28,29 In the present study, the PCL mat with porous fibers has been treated with alkali to hydrolyze the surface of the fibers in order to improve cell adhesion property and faster degradation. The PPCL mats degrade many folds faster than normal PCL mats (data not shown) under in vitro conditions.
Another potential application of the PPCL-coculture is to use it as an organotypic system. Three dimensional organotypic skin models closely mimic the natural skin and therefore is a suitable alternative for testing in animals. Generally a skin model consists of primary keratinocytes on collagen gel dispersed with dermal fibroblasts. The self organized coculture of keratinocyte and fibroblasts on PPCL could be an attractive in vitro model for toxicity testing
Skin substitutes act as a covering over the wound and aid to restore the structure and function of the skin. We have demonstrated the feasibility of using PPCL electrospun mats to coculture keratinocytes and hair follicle derived dermal fibroblast cells, towards potential use as a skin substitute. The results obtained showed PPCL mat as a good substrate for cell adhesion and favored self organization of cells. The keratinocytes formed multi layers analogous to the epidermis while the fibroblast showed a more dispersed pattern with migration of cells into the mat, similar to the dermis. The presence of porous fibers enhances the cell adhesion area and faster degradation due to increased surface area. Furthermore, the proposed coculture system is a promising skin equivalent that can serve as an in vitro toxicity system.
Authors acknowledge the financial support to Anil Kumar PR (Grant #: SR/FT/LS-128/2009) and Kumary TV (Grant #: SR/SO/HS-0042/2012) from Department of Science and Technology, Government of India.
The authors have no conflict of interest or competing financial interests.

©2017 Madathil, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.