Advances in
eISSN: 2572-8490


Commentary Volume 2 Issue 5
SI Institute of Neurosurgery n. acad. A. Romodanov NAMS of Ukraine, Ukraine
Correspondence: Oleksenko NP, SI "Institute of Neurosurgery n. acad. A. Romodanov NAMS of Ukraine, Ukraine
Received: May 17, 2017 | Published: August 4, 2017
Citation: Tsymbalyuk VI, Vasylyeva IH, Oleksenko NP. Interaction of components in 3d prf matrix and neuronal cells in vitro. Adv Tissue Eng Regen Med Open Access. 2017;2(5):252–254. DOI: 10.15406/atroa.2017.02.00044
The task of modern neuro transplantology is to find adequate 3D matrix for transplanted cells. The requirements proposed for the 3D matrix in neurotransplantation consist in the following - the absence of toxicity, the possibility of biodegradation, the maintenance of integration and functional activity of targeted transplanted cells into the tissue. Recently, an attention is focused on the matrix obtained using plasma rich fibrin (PRF) to improve the results of stem cell transplantation.1 Studies have shown that the use of fat cells in combination with PRP (platelet-rich plasma) promotes the graft revascularization and improves the survival in transplantation.2,3 It is believed that the PRP properties are determined by platelets that are a source of growth factors containing in the α-granules. Platelets circulate in blood stream in an inactive state until their contact with the damaged endothelium. Platelet activation is accompanied by degranulation with the release of growth factors – counting more than 20. PDGF (platelet-derived growth factor), TGFβ (transforming growth factor beta), IGF (insulin-like growth factor), VEFG (vascular endothelium growth factor) and EGF (epidermal growth factor) are the most important of them.4,5 Angiogenesis, cell proliferation, differentiation and formation of extracellular matrix are stimulated by growth factors. An important component of platelet α-granules is also prothrombin that plays an important role in the platelet aggregation. PRF use allows to obtain the matrix, which 3D structure is provided by thrombin with included platelets, capable to active prolonged release of growth factors.1,4-6 If necessary, the neuronal, mesenchymal or other stem cells can be enclosed in matrix.
This method has the following advantages: fibrin clot can be obtained for 1-2 hours before the need to use; this is autologous material that removes the ethical issues, problems of immune conflicts and infection; this is an inexpensive method. There are few studies where this method is used, but the positive results are emphasized by some authors today.2,3,6,7
In our work 3D PRF matrix was obtained from human venous blood samples8 by centrifugation at 3000 rpm for 10 min using the coagulation activator SiO2 (Vacumed, F.L. Medica Italy) in 10 ml vacutainer. After centrifugation, it settles in three layers, the upper straw-colored acellular plasma, middle portion containing the fibrin clot, and red-colored lower portion containing red blood cells. The upper straw-colored layer is removed and middle portion is collected, 2 mm below to the lower dividing line, which is the PRF. To study the effect of fibrin matrix density on the vital functions of nerve cells and the culture results, 3D PRF of different density (high and low) were used. Their platelet count was 200 and 100 thousand per ml respectively. The suspension of the brain nerve cells of newborn rat was administered into the middle of the clot by injection and was applied to its outer surface under different experimental samples. Culture was carried out in medium DMEM (Dulbecco's modified Eagle's medium) (PAA, Austria), enriched with 10 % serum of FBS (fetal bovine serum) (PAA, Austria) for 10 days at 37˚C and 5% CO2.
Monitoring of nerve cell culture showed the active formation of growth zone, axonal invasion, the establishment of contacts between cells, the formation of confluence in cell layer when cells were applied on the outer surface of 3D PRF matrix (Figure 1A-1C). When cells were administered inside 3D PRF matrix the active cell migration on the outer surface of the matrix, the formation of contacts by cells in the middle of the matrix, and the network of processes forming neurite-glial fibers with their invasion outside were noted. The dense layer is form by the cells (Figure 1D-1F). Partial lysis of fibrin matrix was revealed at the end culturing (Figure 1B).
A clear difference in the rate of axonal invasion through matrix towards the surrounding cells was noted by us. When using 3D matrix with high density this process occurred more slowly (Figure 1G,1H). Thus, our studies have shown that the formation of supportive environment without toxic effect on nerve cells that actively form the network of branches, establishment of contacts and formation of cell layer with high confluence is a result of 3D PRF matrix use. We consider it advisable to continue studying the properties of 3D PRF matrix as a substrate for nerve cell transplantation.

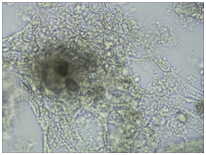



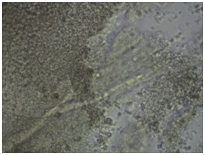

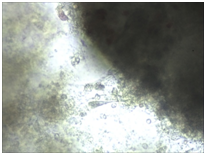
Figure 1 3D PRF matrix of high- and low-density with application of nerve cells suspension in culture condition.
None.
The author declares no conflict of interest.

©2017 Tsymbalyuk, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.