Advances in
eISSN: 2572-8490


Review Article Volume 2 Issue 5
Karlsruhe Institute of Technology, Germany
Correspondence: Eric Gottwald, Institute of Functional Interfaces, Karlsruhe Institute of Technology, Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
Received: June 12, 2017 | Published: July 24, 2017
Citation: Nies C, Gottwald E. Artificial hematopoietic stem cell niches-dimensionality matters. Adv Tissue Eng Regen Med Open Access. 2017;2(5):236–247. DOI: 10.15406/atroa.2017.02.00042
Hematopoietic stem cell niches are perhaps the best described niche system in mammals. The niches themselves, as well as their cellular and structural constituents and factors that play a role in maintaining the niche structure and function, are being refined on a more or less daily basis. Despite this, it seems as if the more we know the harder it gets to mimic the in vivo-situation using in vitro-systems. This is due to the fact that hematopoiesis is a multi step maturation process leading to HSC heterogeneity. Subpopulations of HSCs and niche supporting cells can be defined depending on characteristics such as their potency of leading to successful reconstitution of sub lethally irradiated mice in serial transplantation experiments or, with less scientific impact, clonogenic assays. Since the bone marrow obviously provides all necessary information to maintain the stem cell pool constant and to adapt the number of blood cells according to physiologic needs, it has been the goal to engineer artificial niches that display at least one or several of the major characteristics of the in vivo-situation to make use of these systems for not only fundamental research purposes but, moreover, also for clinical applications. This review will give an overview of approaches to engineering artificial hematopoietic niches with a focus on the complexity/dimensionality of the systems used.
Keywords: Hematopoietic stem cell; Marker; Differentiation; Niche; Progenitor; Mesenchymal stem cell, Artificial
AC: Adipocytes; AGM: Aorta Gonad Mesonephros Region; ALCAM: Activated Leukocyte Cell Adhesion Molecule; BM: Bone Marrow; BMPRIA: Bone Morphogenetic Protein Receptor Type IA; BFU-E: Burst-Forming Unit-Erythrocyte; CFU-C, -GM, GEMM, -S: Colony-Forming Unit-Granulocytic Progenitor Cells, -Granulocyte-Monocyte, Granulocyte, Erythrocyte, Monocyte/Macrophage, Megakaryocyte, -Spleen; CC: Chondrocytes; CXCL-12: C-X-C-Motif-Chemokine Ligand-12; EHT: Endothelial-To-Hematopoietic Transition; G-CSF: Granulocyte Colony-Stimulating Factor; LTC-IC: Long-Term Culture-Initiating Cells; MDGF: Megakaryocyte-Derived Growth Factor; NMSC: Non-Myelinating Schwann Cells; OB: Osteoblasts; Ocn: Osteocalcin; Osm: Oncostatin M; Osx: Osterix; PDGFR: Platelet Derived Growth Factor Receptor; PDMS: Polydiemthylsiloxane; PTH: Parathyroid Hormone; PVC: Perivascular Stromal Cells; SCF: Stem Cell Factor; SDF-1: Stromal Cell-Derived Factor-1; SEC: Sinusoidal Endothelial Cells; TGF-β: Transforming Growth Factor β
Hematopoiesis is the process that ultimately leads to the generation of all blood cell types. This process in mammals begins with the transdifferentiation of a subpopulation of endothelial cells in the aorta-gonad-mesonephros region (AGM) of the embryo,1,2 a process named endothelial-to-hematopoietic transition (EHT).3 In addition to being in the embryonic ventral aorta, HSCs have also been shown to be in the head,4,5 the placenta6,7 and the yolk sac.8 With the progression of embryonic development, the HSCs migrate to the fetal liver, where they expand and are maintained until shortly before birth, at which point they again migrate and finally reside in the bone marrow in hematopoietic stem cell (HSC)-supportive niches.4,9 The different origins and habitats of HSCs also seem to lead to a significant heterogeneity in their functional properties such as lineage output and self-renewal ability.9 For current and future artificial hematopoietic systems it will be crucial which HSCs are used and/or combined with other niche cell types and under which conditions they are cultivated. In this review, the term hematopoietic stem cell is used for those cells that are able to self-renew and generate all blood cell types while the term hematopoietic progenitor cell refers to cells that have lost the ability to self-renew while still being able to contribute to complete lineage commitment. In the following, some of the important points to consider for engineered hematopoietic niches, such as cell sources, scaffolds, 2D vs. 3D-approaches, and the desired/observed outputs will be discussed.
Hematopoietic niche models
Despite our well-established knowledge that hematopoietic stem cells in adults reside primarily in the bone marrow, we are far from being able to understand fully which cell types, soluble factors, dimensionality, and mechanical cues are responsible for the successful orchestration of a life-long hematopoiesis (Figure 1). To date, several models are able to explain some of the main features that characterize the behavior of HSCs in their niche. The most widely accepted of these are the endosteal niche, the perivascular niche and, recently proposed, the hemosphere niche model which combines the perivascular and endosteal niche models.10 In the following paragraph, the main features of the models shall be highlighted. The evolution of a “niche” concept is connected to the work of Till & McCulloch,11 who demonstrated colony-forming-units in the spleen (CFU-S) after injecting healthy bone marrow cells in supralethally γ-irradiated mice, leading to the notion that “the results are thus compatible with the assumption that single viable cells from the marrow graft are able to give rise to colonies in the spleen” and “because the marrow suspension injected contained many different cell types, the identity of the cells giving rise to colonies is uncertain”.11 By this, not only the tissue stem/progenitor cell concept but also the necessity of “colony-forming-unit”-supporting cells, the niche-building constituents, were implicated. This concept was confirmed later on by Worton et al.12 Then, Dexter and colleagues demonstrated that the crucial feature for the maintenance of CFU-S- and CFU-C-activity was a stromal layer of cells consisting of bone marrow phagocytic mononuclear cells, epithelial cells and giant fat cells.13 It was not until 1978 that Schofield hypothesized that the CFU-S cells were first generation-colony forming cells rather than real stem cells and that stem cells reside in niches that maintain stemness, enable self-renewal, prevent differentiation, and limit the number of stem cells.14 The close linkage of hematopoiesis to bone marrow was shown by Lord et al.15 and Gong16 as well as Calvi et al.17 and Zhang et al.18 who demonstrated that an increase in osteolineage cells by constitutive expression of an activated parathyroid hormone receptor or a bone morphogenetic protein receptor type 1A depletion, respectively, lead to an increase in hematopoietic stem/progenitor cells. All the groups described what today is known as the endosteal niche in contrast to the perivascular niche described by Kiel and colleagues.19 In addition to the two proposed niche compartments, Wang and colleagues introduced a third one which they termed hemosphere. This model unites the two earlier models which identified a zone between sinusoidal endothelial cells (SEC) and perivascular stromal cells (PVC) that contain clusters of HSCs. Deletion of vascular endothelial growth factor receptor-2 (VEGFR2) from endothelial cells disrupted the formation of hemosphere structures and led to reduced numbers of HSCs in bone marrow.10 An overview of the niche compartments is depicted in Figure 2.
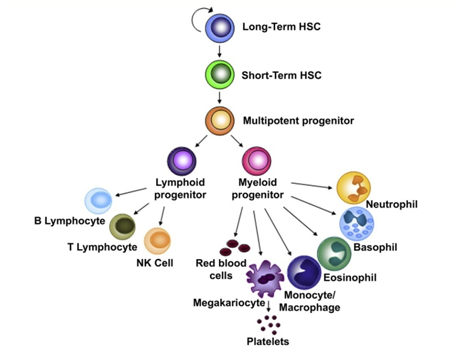
Figure 1 HSC hierarchy. Long-term HSCs (LT-HSC) have the capacity to generate all the blood-cell lineages but they and are also able to assure the maintenance of a stem cell pool through self-renewing divisions [105].
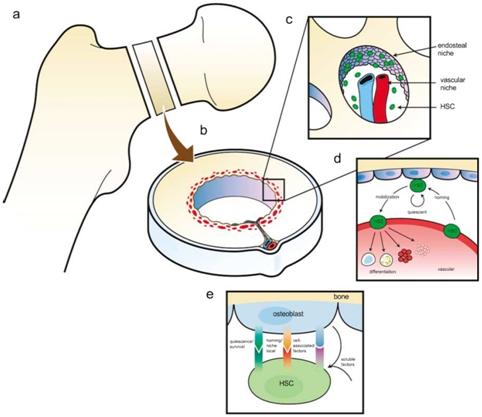
Figure 2 A model of the hematopoietic stem cell (HSC) niche in long bones. (a and b) Organization of long bones and location of the HSC niches. Recent studies demonstrate that endosteal osteoblasts and their precursors play a critical role the stem cell ‘niche’ (c). In addition, endothelial cells are likely to contribute to niche function (c). Central to these hypotheses are the demonstration of osteoblast (OB)- or endothelial cell-expressed regulatory components that influence stem cell function and are likely to include cell-to-cell receptors, and soluble and cell-surface associated cytokines and growth factors. (d) Egress into and out of the marrow by HSCs facilitates transit into and out of the vascular niche, which is permissive for proliferation and differentiation. The endosteal niche facilitates HSC maintenance and quiescence. Reciprocal interactions between stem cells and their niches are likely to play key roles in the establishment and maintenance of the stem cell niche in the bone marrow (not shown). (e) Factors produced by osteoblasts that influence HSCs. Stem cell fate is influenced by specialized microenvironments that remain poorly defined. Osteoblasts produce soluble hematopoietic-supportive secreted and cell-associated factors that work in concert so that HSCs derive regulatory information from bone, accounting for the localization of hematopoiesis in bone marrow (homing/localization receptors e.g. Annexin II, VCAM-1, CXC chemokine receptor 4 (CXCR4)/CXC chemokine ligand 12 (CXCL12)). Quiescence factors (e.g. bone morphogenic factors, fibroblast growth factors, Flt-3 ligand, Tie2/Ang-1, granulocyte and granulocyte-macrophage colony-stimulating factors, hepatocyte growth factor (HGF), leukemia inhibitory factor (LIF), osteopontin and high levels of extracellular calcium) and cell-associated factors are known to include but are limited to osteopontin, granulocyte and granulocyte-macrophage colony-stimulating factors and transforming growth factors (TGFs). Soluble factors known to influence HSC function include parathyroid hormone and erythropoietin (Epo) (reproduced with permission of Nature Publishing group).
Hematopoietic stem cells
Human hematopoietic stem cell sources comprise the bone marrow, peripheral blood, the postpartum placenta and the umbilical cord. Due to their more naïve characteristics and their availability, HSCs from cord blood are widely used in research. However, there are some differences compared to peripheral blood and bone marrow such as differences in the composition, number and properties of the cells.20-24 CD34+CD38-cells, locked in the G0-phase of the cell cycle, show a greater proliferative response to cytokines and are less dependent on stromal cells than the corresponding cells in the bone marrow or peripheral blood.20,24-26 Moreover, cord blood differs from bone marrow and peripheral blood with respect to the number of colony forming cells. Cord blood contains 8.000 BFU-E forming cells, between 13.000 and 24.000 CFU-GM and between 1.000 and 10.000 CFU-GEMM forming cells in 1 ml of blood which is 3 and 15 times more than the numbers in marrow and peripheral blood, respectively.20,27,28 The number of cells expressing the surface antigen CD34 in cord blood lies between 0.02 and 1.43%, which is close to the values in bone marrow cells (0.5-5%) but different from peripheral blood (<0.01%).20,29 Also, the number of CD34+HLA-DR-cells and CD34+CD38-cells in cord blood is higher than in bone marrow (4% compared to 1%).20,30 Adhesion receptors play a major role in the hematopoietic process. More than 20 different adhesion receptors have been identified on hematopoietic progenitors.31 The expression of integrins on CD34 expressing cord blood cells is higher than for CD44, CD49d, and CD49f but lower inCD11 and CD18 compared to bone marrow or peripheral blood.25 The more naïve character of the cord blood cells can also be explained by the longer telomere length which is responsible for the larger number of progeny.20,24,28 Inside and outside the niche(s) a cocktail of cytokines is constantly sensed by the cells. Cord blood HSCs seem to have a higher affinity for stromal cell-derived factor-1 (SDF-1), react stronger to IL-3, IL-6 and stem cell factor (SCF) leading to a larger number of colonies.20,32-34
When engineering niches it is therefore important to keep these differences in HSC sources in mind and to adapt the experimental conditions accordingly.
Bone marrow niche supporting cells
To name all other cellular components of the bone marrow niche is quite a task since the identification of the cell types involved sometimes refers to function, location, marker expression, differentiation stage or even combinations of these features, we therefore, will stick to the convention of Ugarte and Forsberg10 who proposed to use the term “stromal cells” for all non-hematopoietic cell types (CD45-Ter119- or CD45-Lin-) such as mesenchymal stromal cells (MSC), perivascular cells (PVC), (sinusoidal) endothelial cells ((S)EC), osteoblasts (OB), adipocytes (AC), chondrocytes (CC), and non-myelinating Schwann cells (NMSC). Moreover, Ugarte and Forsberg proposed to group the cells according to lineage relationships which leads to the use of OBCs for the osteoblastic lineage including osteoprogenitors and osteoblasts as well the term MSPC for mesenchymal stromal progenitor cells as proposed by Frenette et al.35 For reference of the cellular composition of the bone marrow niche Table 1.
Cell Type |
Identity/Markersa |
Mouse Modelb |
Niche Function |
Perivascular Stromal Cell |
CXCl12hi |
Nestin-GFP [43,45] |
HSC maintenance and retention in BM |
-Nes-GFP |
Pdgfr-a |
Prxf -cre [44,46] |
Direct supply of soluble and non-soluble factors for HSCs |
-SCF GFP |
Pdgfr-b |
Cxcll2-GFP [42]/DsRed [46] |
|
-Cxc112-GFf /DsRed(CAR) |
CD146 |
Cxcll2-DTR-GFP [49]/ Osx-cre [44] |
Precursors of other niche cells-osteoblasts, adipocytes, chondrocytes, etc. |
CFU-F Potential Mesenchymal Lineage differentiation |
Lepr-cre [45,46]/ Scf-GFP [45] |
||
Sinusoidal Endothelial Cells |
CD31 |
Tie2-cre [44-46]/Cdh5-CreER [5] |
HSC maintenance |
Osteoprogenitors |
Osterix |
Osx-cre [44], |
Lymphoid progenitor cell niche |
Osteoblast (OB) |
Osteocalcin, |
Prx1-cre [44,46], Osteocalcin-cre [44], Osx-cre [44,46] |
|
Adipocytes |
Fabp4 |
A-ZIP/F1 [60] |
Negative regulators of haematopoiesis |
Monocytes/Macrophages |
Gr-1 |
CD169-DTR [59] |
Regulating MSC and OB Function. Active player in G-CSF mobilization. |
CD169 |
Gr-1-DTR [59] |
||
CD11b |
MAFIA [55,59] |
||
F4/80 |
Clodronate Liposomes [55,59], CD68:G-CSFR [44] |
||
Non-myelinating Schwann Cells |
Active TGF-B |
Tgfbr2fl/- [47] |
Maintenance of HSC Quiescence |
Table 1 Non-hematopoietic niche cell components (reproduced with permission of WileyVCH Verlag GmbH & Co. KGaA).
aCommonly used markers to describe the respective cell population.
bMouse models used to interrogate the respective cell population.
Since the stromal cell composition has been expertly reviewed by Yu & Scadden,36 Lo Celso & Scadden,37 Ugarte & Forsberg,10 and Nakamura et al.,38,30 I will only briefly summarize the non-hematopoietic cellular composition of the bone marrow niche.
Osteolineage cells
Perhaps the most contradictory results with regard to the function of osteolineage cells within the niche has been reported about these cell types. By conditional deletion of the bone morphogenetic protein receptor 1A (BMPR1A) in hematopoietic cells using the Mx-1-cre promotor system, it was shown that genetic intervention of osteolineage cells alters the number of HSCs and downstream hematopoiesis.18,36 Similar results were obtained by osteoblast-specific expression of the parathyroid hormone receptor (PTH).17,36 However, since the osteoblastic population shows a diverse marker expression, it was shown that major ablation of a sub-population of osterix (Osx) and osteocalcin (Ocn) positive cells does not interfere with long-term HSC maintenance but instead reduces T-competent progenitors in the thymus.36,39 Other sub-populations expressing activated leukocyte cell-adhesion molecule (ALCAM) and/or stem cell antigen-1 (Sca-1) maintained long-term reconstitution activity of HSCs in vitro in which ALCAM+Sca-1--cells enhanced LTR-activity by upregulation of homing- and adhesion-related-genes, while more mesenchymal lineage-like cells (ALCAM-Sca-1+) highly expressed cytokine-related genes indicating that these cells are involved in the regulation of HSC proliferation and quiescence.38 Another marker shown to be dependent on the differentiation stage of the ostelineage cell is the ribonuclease DICER. Whereas conditional deletion of DICER in osteoprogenitors dramatically affects hematopoiesis, no effect was observed in mature osteocalcin+ osteoblasts.37,40
Perivascular (stromal) cells
Due to their localization, perivascular cells have been early “hot” candidates for being necessary to maintain HSC homeostasis. Within this population, the best studied cell types are the mesenchymal stromal cells and the C-X-C-motif-chemokine ligand-12 (CXCL-12) abundant reticular cells (CAR), although the latter have been proposed to be a subpopulation of MSCs.40 The cells are responsible for the retention in the bone marrow,42,45 the direct supply of soluble and non-soluble factors,42,44 their capacity to differentiate into other niche cell types such as osteoblasts, adipocytes and chondrocytes,44-46 as well as the maintenance of HSC quiescence.47 Some of the functions have been associated to markers such as CD146,48 CXCL-12, SCF,43,45,46 platelet derived growth factor receptor (PDGFR)-α and –β44,49 and Sca-1,50 stem cell factor (SCF),43 and CD51.51,30 Meanwhile, it seems to be clear that mesenchymal stromal cells are the key players among the non-hematopoietic cells in the niche.
Endothelial cells
The bone marrow is a highly vascularized structure and it is generally agreed upon that HSCs reside in close proximity to the bone vasculature. The endothelial cells of the bone marrow are believed to have a range of effects on HSCs such as the provision of adhesion molecules (selectins, vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule 1),52 lineage specific cytokines (G-CSF, GM-CSF, M-CSF, Kit-ligand, IL6, FLK-2 ligand, leukemia inhibitory factor53), and Notch ligand.54 Disruption of endothelial cell function has been conducted by many groups with more or less severe effects on HSC fate. Blocking E-selectin after chemotherapy and irradiation led to an improved HSC survival,36,55 and conditional deletion of Tie2+ endothelial cells reduced HSC numbers without inducing stem cell mobilization.36,44 Altogether, the data raised so far indicate a prominent role of endothelial cells in the niche.
Monocytes/macrophages
This lineage is indispensable for the niche because of its capability of orchestrating the interplay between other niche cells and the HSCs. The function of the monocytes/macrophages is believed to be connected to the influence on mesenchymal stromal cells and osteoblasts. Albiero et al.56 showed that CD169 labels M1 macrophages and that conditioned medium from M1 macrophages, but not from M0 and M2 macrophages, induced CXCL12 expression by mesenchymal stem/stromal cells. In silico data mining and in vitro validation identified oncostatin M (OSM) as the soluble mediator contained in M1conditioned medium that induces CXCL12 expression via a mitogen-activated protein kinase kinase-p38-signal transducer and activator of a transcription 3-dependent pathway. Moreover, conditional depletion models showed that reductions in BM mononuclear phagocytes led to reduced bone marrow CXCL12 levels, the selective down-regulation of HSC retention genes in Nestin+-niche cells, and egress of HSCs/progenitors to the bloodstream. Of these, depletion of CD169+ macrophages were sufficient to induce HSC/progenitor egress. The macrophage depletion also led to an enhanced mobilization of HSCs by C-X-C-chemokine receptor type 4 (CXCR4)-antagonists or granulocyte colony stimulating factor (G-CSF) implicating a tightly controlled signaling network with the sympathetic nervous system since the sympathetic neural tone is crucial for both steady state57 and granulocyte colony-stimulating factor (G-CSF)-enforced release58 of HSCs/progenitors from the bone marrow.59
Adipocytes
Another cell type that is part of the niche and involved in the regulation of hematopoiesis is the adipocyte. Naveiras et al.60 showed that hematopoietic stem cells and short-term progenitors are reduced in frequency in the adipocyte-rich vertebrae of the mouse tail relative to the adipocyte-free vertebrae of the thorax and that in lipoatrophic A-ZIP/F1 ‘fatless’ mice treated with the peroxisome proliferator-activated receptor-c inhibitor bisphenol A diglycidyl ether, marrow engraftment after irradiation is accelerated relative to wild-type or untreated mice which indicates that adipocytes act as predominantly negative regulators of the bone-marrow microenvironment.
Sympathetic nervous system cells
It has been shown that the sympathetic nervous system is regulating the hematopoietic niche function as well, directly and indirectly.61 The direct mechanism is related to adrenergic stimulation of osteoblasts. Lee-Thedieck et al.62 showed that the stiffening of osteoblasts was due to a decreased F- to G-actin ratio leading to a reorganization of the cytoskeleton. The indirect mechanism is related to the sensing of light. HSCs and their progenitors exhibit robust circadian fluctuations peaking 5 h after the initiation of light exposure and reaching a nadir 5 h after darkness.57 This effect was due to noradrenalin secretion by the sympathetic nervous system leading to the antiphasic expression of CXCL-12 on stromal cells. This study also revealed that the adrenergic signals are locally delivered by nerves of the bone marrow and transmitted to stromal cells by the β3-adrenergic receptor which leads to a decreased nuclear content of Sp1 transcription factor and rapid down regulation of CXCL-12 expression.20,57 This signaling cascade was further characterized by Yamazaki and colleagues who showed that Schwann cells are responsible for maintaining HSC hibernation.36 Mice displaying autonomic nerve denervation, reduced number of TGF-β-producing cells or TGF-β type II receptor deficient HSCs exhibited reduced levels of Smad activation in HSCS, loss of HSCs from the bone marrow, and impaired long-term repopulation activity .36,47 In addition, Katayama and colleagues demonstrated that UDP-galactose ceramide: galactosyl transferase-deficient (Cgt-/-) mice exhibited aberrant nerve conduction and displayed no HSPC egress from the bone marrow following G-CSF or fucoidan administration.36,58
As we have learned from the previous sections, a multitude of cells as well as a multitude of factors are needed to maintain proper niche function, so that it is hard to summarize all the approaches for engineering an artificial niche with respect to “proper functioning”. Moreover, mouse and human niches differ in some respects so that the cellular composition of artificial niches realizable in the mouse setting may not be transferable to the human situation. In addition, the extracellular matrix composition influences HSC differentiation, proliferation, lineage specification, and apoptosis.63-66 The materials used in studies are therefore of utmost importance, and, as has been shown for some materials, may not be suitable for the in vitro culture of CD34+ cells.67 However, in an attempt to give an overview of current artificial hematopoietic niches, we will approach the topic by taking into account the system complexity. Beginning with suspension culture, and two-dimensional systems, we will move forward to single cell- and 3D- (multicellular) systems. Heterogeneity is further increased by implementing flow and other capabilities such as high-throughput or upscaling capabilities. Last but not least, even an in silico approach will be discussed.
Suspension culture
One of the simplest culture methods of all for HSCs is the suspension culture technique. Harvested cells are put into medium, usually supplemented with a cocktail of growth factors and cytokines, and cultured for the desired period of time. The culture vessel can be a classical one such as a Petri dishor a flask68 or, for clinical applications, be bags. With the former approach it has been shown that medium containing KIT ligand, FLK2/FLT3 ligand, interleukin-6 (IL-6), and erythropoietin, with or without IL-3, CD34+c-kitlow-cells after a culture period of 2 weeks showed a day-60 engraftment capability whereas long-term engraftment failed.69 With the bag approach, it was shown that CD34+ cell expansion prior to transplantation is feasible.70,30 For this, the cells were cultured for 10 days in ateflon coated propylene bag containing 800 mL of defined media and 100 ng/mL each of SCF, G-CSF, and megakaryocyte-derived growth factor (MGDF).
Two-dimensional, multi-cell-systems
The next step in increasing the complexity is the increase in cell number and/or the co-culturing of one or more cell types, moving the system further towards the in vivo-situation but at the same time excluding monocausality. The expansion potential of hematopoietic stem and progenitor cells was analyzed using 6-well plates coated with tropoelastin. A two- to three-fold expansion with a further increase after addition of IL-3, IL-6 and mouse stem cell factor could be observed. Since neither a truncated tropoelastin nor a cross-linked one was able to reproduce this effect, elasticity and tensegrity seemed to play an important role in HSC expansion.71 Lee-Thedieck et al.62 used flat fibronectin hydrogels to analyze the reaction of the hematopoietic line KG-1a and HSCs from cord blood with regard to adhesion and migration. They found that the number of adhering KG-1a cells or HSCs was higher and the cells migrated faster on hard gels. The culture of cord blood HSCs on top of an irradiated (20 Gy) mesenchymal stem cell (MSC) feeder layer maintained a primitive immunophenotype (CD34+CD133+ and CD38-) for more population doublings, while up-regulation of differentiation markers (CD13, CD45 and CD56) in HSCs was delayed to higher numbers of cell divisions. In particular, MSCs of early cell passages maintained CD34 expression in HPC over more cell divisions, whereas MSCs of higher passages further enhanced their proliferation rate.72 Similar results were obtained by cultivating HSCs and MSCs long term in 2D with concomitant transplantation in mice. It was shown that the capacity of some totipotent hematopoietic stem cells can be maintained and amplified over extensive time periods in vitro without diminution of their long-term in vivo-repopulating potential as judged by competitive repopulating unit numbers.73,30 PET films modified with connecting segment-1 (CS-1) and RGD-motifs were used for expansion purposes. The highest CD34+ cell expansion was observed on the CS-1 peptide-modified surface, where total nucleated cells, total colony forming unit, and long-term culture initiating cells were expanded by 589.67 ± 58.6 (mean ± s.d.), 76.5 ± 8.8, and 3.2 ± 0.9-fold, respectively.74 After 10days of ex vivo expansion, only cells cultured on a CS-1- immobilized surface yielded positive engraftment. With the aim of retroviral transduction of CD34+ cells from peripheral blood or bone marrow, only cultivation with stromal cells led to a successful transduction of the latter whereas those of the peripheral blood could be transduced in the presence of either cytokines or stroma.75
Single cell systems
The single cell systems used for the cultivation of HSCs are somewhat hard to categorize. This is due to the fact that the systems themselves offer a more or less 3D or “quasi-3D” environment but, due to the fact that single cells are incorporated, lack a real 3D organization. However, single cell systems offer some advantages over more complex systems such as the possibility for a more stringent cause-effect-analysis due to the limited components typically involved. Single cell systems have therefore been widely used to characterize single parameters in HSC behavior such as adhesion or stem cell maintenance. Dykstra and colleagues manufactured single cell microwells based on silicone gels for time-lapse video monitoring of HSCs that allowed high-resolution real-time tracking of cells in multiple expanding clones in vitro that could be coupled with functional assays of the individually harvested clones at the end of the monitoring period. They identified two parameters that each showed a significant association with clones containing HSCs after 4 days of culture: a prolonged cell-cycle time measured over three divisions and a reduced proportion of progeny with uropodia at any time between 84 and 96 h of culture.76 In an attempt to dissect the impact of geometrical constraints and adhesive interactions on HSCs during cytokine-driven expansion, Kurth et al.77,78 used CD133+-cells that were cultivated in micrometer-scale cavities with 10 µm in depth and 15 to 80 µm in diameter, manufactured from PDMS and coated with fibronectin or collagen I. In this model it was shown that HSCs residing in smaller cavities displayed a decreased proliferation and differentiation accompanied by decreased DNA synthesis and an increased HSC marker expression.77 Similar results have been shown with another system described as a single cell-system, although the characteristics of the system are also suited to be mentioned under 3D-systems. Based on PEG-hydrogels, Lutolf et al.79 could show that those HSCs residing in microwells coated with Wnt3a or N-Cadher in displayed reduced proliferation kinetics and increased asynchronous division and that these cells were able to reconstitute whole blood in serial transplantation experiments in mice.80 A device comprising a pneumatic valve which enabled the culturing of different types of niche cells in different parts of the same device was developed.81 Single HSCs could be injected into the microfluidic device, manipulated, and placed onto different niches within the same device as controlled by the user.
Synthetic, three-dimensional, Multi-Cell-Systems
Over the last decades is has been impressively demonstrated that three-dimensionality is an essential characteristic of organotypic behavior, be it simple morphology or more complex parameters such as stem cell maintenance, multipotency or even resistance to drugs. Inclusion of the next dimension in in vitro-models is therefore a logical consequence for the analysis of the above mentioned parameters. Figure 3 provides a synopsis of the approaches described below.
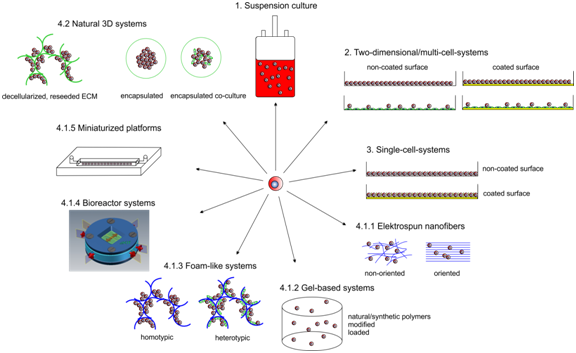
Figure 3 Synopsis of artificial hematopoietic niche approaches. Enumeration according to paragraph 4 of the text. Yellow lines. surface modification, blue lines: polymer/artificial scaffolds, green lines: natural scaffolds
Electrospun nanofibres
One system that is popular not only for modelling the HSC niche makes use of electrospun nanofibers. Chua et al.82,83 used such a system for expansion purposes. They found that aminated nanofiber meshes were most efficient as compared to tissue culture polystyrene (195 fold and 50 fold, respectively). Das and colleagues and Lu and colleagues have used a modified polyether sulfone nanofiber system for expansion of CD133+ cord blood cells.84,85 Other expansion 3D-approaches made use of non-woven polyethylene terephthalate (PET). CD34+ cells from cord blood cultured in these structures showed a 30 to 100% increase in total cell number without the addition of cytokines. With thrombopoietin and flt-3/flk-2 ligand a two- to three-fold higher total cell number was demonstrated compared to 2D culture.86 3D-PET scaffolds were also used when surface modified with fibronectin. The study demonstrated the synergistic effect between the three-dimensionality of the scaffold and surface-conjugated FN and the potential of this FN-conjugated 3D scaffold for ex vivo-expansion of HSPCs.87
Gel-based systems
Gel-based systems also showed promising results with regard to multiplication and preservation of stemness. Raic et al.88 could show that by employing a salt leaching technique to prepare porous poly (ethylene glycol) diacrylate (PEGDA) hydrogels, HSCs from cord blood in co-culture with mesenchymal stromal cells from bone marrow had the strongest proliferative effect and that this effect was due to the 3D-environmentwhen compared to a 2D-system. Using collagen gels, with or without embedded MSCs, only HSCs from bone marrow could be shown to preserve stemness whereas HSCs from cord blood displayed increased proliferation and lineage commitment.89 Stem cell factor coupled to methacrylamide-functionalized gelatin gels (GelMA) has been used to analyze the influence on Lin−Sca-1+c-kit+ (LSK) cells with regard to maintaining more primitive HSC fractions, viability and expansion. It was shown that matrix-immobilized SCF is more selective in its ability to maintain primitive HSC fractions within a fully-3D hydrogel biomaterial as compared to soluble SCF in the media, likely due to exclusion of soluble SCF-induced differentiation.90 A unique approach that made use of so-called inverted colloidal crystals (ICC) for the generation of polyacrylamide gels with a layer-by-layer-surface coating of DLL-1 notch ligand showed that when cultivating the mononuclear fraction, pre-erythrocytes and dendritic cells could be observed whereas when HSCs from cord blood were cultivated, T-cell lineage commitment and differentiation could be seen.91
Foam-like systems
Scaffolds of different materials have also been employed for various purposes. For preserving the stem cell character as well as for the production of progeny, polyurethane foams (PFU) were used.92 It could be demonstrated that 3D-cultures in different PFUs were superior with regard to both parameters, although the study used the whole MNC fraction instead of HSCs, indicating also the importance of stromal cells. Another foam-scaffold approach used poly (L)-lactic acid (PLLA) to differentiate hematopoietic progenitors from mouse embryonic stem cells either alone or in co-culture with a stromal cell line (OP9). In this study it was shown that decreasing the scaffold pore size from 500 to 250 µm increases hematopoietic differentiation of embryonic stem cells (ESC). In addition, an increase in polymer concentration resulted in an increased scaffold compression modulus with a concomitant significantly enhanced hematopoiesis. Finally, higher cell seeding densities as well as co-culture with marrow-derived stromal cells increased HPC generation.93 Poly (L)-lactic co-glycolic acid has been used as an in vitro-model for HSC and MSC homing and engraftment and tissue engineering purposes.94 So-called micromarrows, an HSC/MSC co-culture model based on plates containing microwells whose surfaces have been modified by multi layering of chitosan and hyaluronic acid, showed that the number of HSCs in the system could be doubled relative to monolayer culture and that they expressed higher levels of key hematopoietic niche factors.95 A famous material for bone regeneration, hydroxyapatite, has also been used for bone marrow regeneration using bone marrow stromal cells.96 It was demonstrated that regenerated BM is capable of supporting hematopoietic stem cells (HSCs) and delivering HSCs to native BM in vivo. Carbon-based porous materials (tantalum-coated porous carbon lattices, TCPB) have been employed to analyze HSC maintenance and expansion.97,98 These materials were capable of supporting the maintenance of immature progenitors for up to 6 weeks in the absence of supplemented cytokines. The results demonstrated further that the TCPB matrix facilitated and enhanced HPC maintenance and led to a 1.5-fold expansion of HPC numbers following 1 week in culture and a 6.7-foldincrease in colony-forming ability following 6 weeks in culture in the absence of exogenous cytokines.
Bioreactor systems
Several bioreactor platforms have also been used to cultivate HSCs with or without stromal cell contribution. Meissner et al.99 used a fixed bed bioreactor filled with porous glass carriers to cultivate HSCs with MSCs. They could observe the expansion of very early progenitor cells (CFU-GEMM) up to 4.2-fold and later progenitor cells, CFU-GM and BFU-E, up to 7-fold and 1.8-fold, respectively.A stirred tank bioreactor was used to characterize the kinetics of stem cell factor receptor (c-kit) internalization by human Mo7e cells exposed to different extracellular concentrations of soluble SF,100 and a rotating wall vessel as well as continuously perfused cultures were used for expansion purposes.101,101 A further bioreactor platform was used to co-cultivate mesenchymal stromal cells together with CD34+ cells from cord blood. A micro cavity array comprising 3x105 MSCs and 2x105 HSCs was cultivated under flow conditions (400µl/min) for 14 days. Immunofluorescence, gene expression profiling with sqRT2-PCR and western blots revealed that the HSCs in the bioreactor throughout the entire cultivation period expressed CD34 whereas the expression was lost in control mono layers after 5 days indicating stem cell maintenance in the microcavity array system.103 After a culture period of 21 days, it could be shown by single cell tracking and quantification of HSCs inside single microcavities that HSCs migrate in the cellular network of MSCs where they form two populations, one that resides in the upper region of the microcavity and one that migrates to the bottom of the microcavity where extensive proliferation can be observed and at the same time maintaining CD34-expression (unpublished results, Figure 4). A bioreactor device with a fluid reservoir oscillating between two columns filled a porous material (polyethylene glycol terephthalate, PEGT) and polybutylene terephthalate (PBT), nonwoven meshes of esterified hyaluronan or porous β-tri-calciumphosphate ceramics showed that when medium was supplemented with hematopoietic growth factors not only mature hematopoietic cells, but also early multipotent progenitors (colony forming unit-granulocytes, -erythrocytes, -macrophages, -megakaryocytes, and granulocyte, erythrocyte, monocyte/macrophage (CFU-G, E, M, MK, GEMM)) could be entrapped in the pores of the scaffold.104 This setup was then used by first loading the columns with stromal cells to generate a pre-formed niche and then infusing trackable HSCs. This study showed that after 3 weeks, a subpopulation of HSCs, able to form colonies in vitro and spleen colonies in irradiated mice, was still present.105 Even commercial systems have been used to expand umbilical cord blood. A cassette type bioreactor, constantly perfused with fresh medium,was used to expand umbilical cord blood units during a culture period of 12 days. It could be shown that the median fold increase was 2.4 (range, 1.0-8.5) in nucleated cells, 82 (range, 4.6-266.4) in CFU granulocyte-macrophages, and 0.5 (range, 0.09-2.45) in CD34 lineage negative (lin-) cells [106].

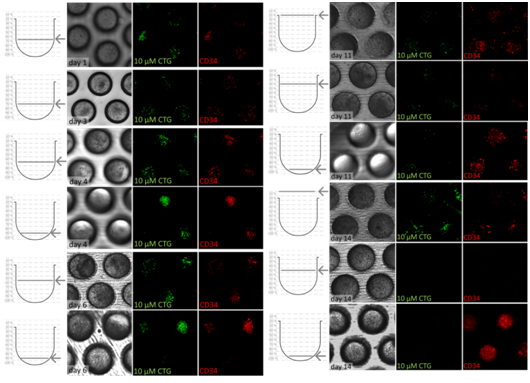
Figure 4 c) Bright field and confocal microscopy image series of 3 microcavities with CD34+ cells stained with Cell Tracker Green and with anti-CD34-antibody (red = phycoerythrin) over a time period of 14 days. Left row: height within the microcavity in which the photo was taken, middle row bright field image of the co-culture, two rows on the right, respectively: confocal images of CD34+ cells stained with Cell Tracker Green and mouse anti-human-PE (red) [119]. The images show the distribution/migration of CD34+ cells within the microcavities with a more regular distribution of cells in the beginning and a migration towards the bottom where extensive proliferation takes place until the end of the experiment.
Miniaturized 2.5 - 3D-platforms
On the one hand, miniaturized platforms offer great potential with regard to parallelization; on the other hand, downsizing of structures below the multicellular aggregate level (< 100 µm) leads to the collection of more than one cell per structure but prevents building real multicellular aggregates such as spheroids or organoids. Therefore, due to the fact that these platforms are neither true 2D- nor 3D-platforms, they should be termed 2.5 D systems. In such a system, Torisawa et al.107 engineered a bone marrow-on-a-chip-platform. For this, a “bone inducing material” was subcutaneously implanted in mice for 8 weeks. After removal, the so-called engineered bone marrow (eBM) was transferred to a chip where it retained hematopoietic stem and progenitor cells in normal in vivo-like proportions for at least 1 week in culture, and organ-level marrow toxicity responses and protective effects of radiation countermeasure drugs could be modelled. A system of interest, although not a true niche model, was described in the study that came up with a microfluidic system that can trap 440 individual HSCs in 18 x 18 x 10 µm cell traps. The device, made of PDMS, was used to characterize T-cell signaling and the signaling dynamics of patient-derived hematopoietic stem cells.108,109 Another study used a device consisting of a microscope slide that was covered with a microscope cover slip with two electrodes at the end that delivered electric fields with a frequency of 1 MHz at a voltage of 20 Vpk-pk. It was possible with this device to direct the cells to the highest field strengths where they could be trapped by infusion of fibrinogen and thrombin. By layering Jurkat cells on top of mouse stromal cells (AC3) and/or SAOS-2 osteoblasts, multilayered constructs that resembled the natural “hematon” could be generated. It has been demonstrated that hemispherical structures with distributions of cells similar to that in the hematon can be made using positive dielectrophoresis.110 In an attempt to recreate the perivascular niche using a microfluidic approach, Carrion and colleagues111 suspended endothelial cells within 3D fibrin gels patterned adjacent to stromal cells (either fibroblasts or bone marrow-derived MSCs). They executed a morphogenetic process akin to vasculogenesis, forming a primitive vascular plexus and maturing it into a robust capillary network with hollow, well-defined lumens. Both MSCs and fibroblasts formed pericytic associations with the ECs but promoted capillary morphogenesis with distinct kinetics. Biochemical assays within the niche revealed that the perivascular association of MSCs required interaction between their α6β1-integrin receptor and EC-deposited laminin.111
Natural and modified natural materials have been employed in addition to synthetic materials to provide a 3D-housing for the culture of hematopoietic cells. In one example, a construct termed bio-derived cancellous bone was used. This deproteinized and partially demineralized bone was processed in small pieces (1 x 0.5 x 0.5 cm) and seeded with mesenchymal stromal cells. Seeding of cord blood CD34+ cells after 2 weeks resulted in an increase in CD34+ cell number (3.3-4.8 fold). CD34+/CD38-cells accounted for 82-90% of CD34+cells. After 5 weeks, the CD34+/CD38-cells in the 3D system increased when compared with initial numbers. The CFU progenitors were more abundant in the 3D-system compared to the 2D-system (4.6-9.3 fold vs. 1.0-1.5 fold) after 2 weeks in culture, and the colony distribution in the3D-system showed a higher percentage of BFU-E and CFU-GEMM, whereas the 2D-system was mainly comprised of CFU-GM colonies. The LTC-ICs in the 3D-system showed 5.2-7.2 fold increase over 2 weeks and maintained the immature state of the hematopoietic progenitor cells (HPCs) over 5 weeks.102 In an attempt to generate blood progenitor cells, agarose was modified with vascular endothelial growth factor. Mouse ESCs were encapsulated in the gel and cultured for 7 days. In the presence of bone morphogenetic protein-4 (BMP-4), cells exposed to immobilized vascular endothelial growth factor α (VEGFα) upregulated mesodermal markers, brachyury and VEGF receptor 2 (T+VEGFR2+) by day 4, and expressed CD34 and CD41 (CD34+CD41+) on day 7. It was found that immobilized VEGFα treatment was more efficient at inducing blood progenitors (including colony forming cells) on a per molecule basis than soluble VEGFα.113 Tiwari et al.114 used bone marrow derived stromal MS-5 cells to grow scaffolds under different culture conditions and 20% O2 and with or without osteogenic medium (Matrices MX1 to MX4). By decellularizing the culture and drying the scaffolds they generated a matrix for CD34+ cells from umbilical cord blood and performed HSC expansion experiments with the four different matrices. HSPCs could be expanded up to 80-fold with differences in primitive marker expression depending on O2-concentration during the scaffold generation culture. In an even more sophisticated approach, Prewitz et al.115 stabilized ECM secreted by MSCs over a period of ten days by glass-immobilized maleic anhydride copolymer, functionalized with fibronectin to anchor the extracellular matrix molecules. By this procedure, after decellularization, a stable extracellular matrix could be produced. Compared to plasma-treated tissue culture plastic controls, osteogenic type bone marrow ECM and collagen-rich ECM was able to expand the CD34+ cell pool by factor of 2 to 3 and showed positive long-term engraftment in NSG mice. In a different approach, Lai et al.116 produced a bone-like matrix by encapsulating mouse MSCs (mMSC) in a collagen matrix and subjected the cells to osteogenic differentiation. After three weeks the matrix thus built was decellularized with sodium deoxycholate and reseeded with MSCs and HSCs. It was shown that compared to pure collagen scaffolds, the number of HSCs and the number of closely interacting HSC-MSC pairs in the bone-like matrix was increased.
Although the simple location of stem cells is not sufficient to define a niche,117 an in silico approach for the recapitulation of HSC homeostasis has been made. For this, a niche with a fixed number of stem cells, capable of producing differentiated cells, and of being able to recover from injury or disease, was modeled.
Two assumptions were implemented,
In an attempt to describe some of the approaches that have been made to gain insight into hematopoiesis and to design artificial niches it seems impossible to engineer “the niche”. Instead, at least in the medium term, artificial hematopoietic systems will be specialized systems with some being superior to others in terms of one or more characteristics. However, the field is rapidly advancing and perhaps hematopoiesis can be pinned down to a very few but essential factors or properties that, when applied in the right concentration, at the right time point, will enable us to control hematopoietic stem cell behavior as desired.
We would like to thank Patrick Wuchter and Kathryn Melzak for critically reading the manuscript and for language editing. Also, I would like to thank Vera Colditz for preparing Figure 4. We are aware of the fact there is far more research out there related to this field but could not mention it due to space constraints. We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of Karlsruhe Institute of Technology.
No potential conflicts of interest, No financial support.

©2017 Nies, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.