Advances in
eISSN: 2572-8490


Research Article Volume 1 Issue 3
1Bahrain Polytechnic, Isa Town, Kingdom of Bahrain, Bahrain
2Department of Materials Science & Engineering, University of Pennsylvania, USA
3Department of Bioengineering, University of Pennsylvania, Philadelphia, USA
Correspondence: Toworfe GK, Bahrain Polytechnic, Isa Town, Kingdom of Bahrain, Bahrain, Tel 97333290457
Received: October 06, 2016 | Published: December 30, 2016
Citation: Toworfe GK, Composto RJ, Ducheyne. Equibiaxial mechano-elastic strain on osteblasts: theoretical considerations. Adv Tissue Eng Regen Med Open Access. 2016;1(3):89–96. DOI: 10.15406/atroa.2016.01.00015
This study outlines a theoretical model that governs how the transmission of mechano-stimulation affects the proliferation of osteoblast-like (MC3T3-E1) bone cells attached to a deformable silasticnano-bioactive membrane. The nano-bioactive silastic membrane was functionalized by exposure to ultraviolet oven (UV Ozone) treatment regimes, coated with an extra-cellular matrix (ECM) protein, which is fibronectin (FN) and then seeded with MC3T3-E1 cells. The proliferation of MC3T3-E1 cells were evaluated after the cells were plated on the functionalized FN-coated nano-bioactive membrane surfaces and subjected to a 2 hour mechano-stimulation comprising of the application of a 2% dynamic equi-biaxial strain at 1 Hz frequency cycle. Elevation of the phalloidin-stained cellular actin cytoskeleton was observed, indicating the occurrence of mechanical transduction of the applied strains through the cell integrin receptors which culminated in the reorganization of the cellular cytoskeleton. The classical theory of elastic membrane deformation, therefore, rightly predicts mechano-transduction between the bioengineered nano-bioactive membrane surfaces and the applied mechanical force strains, via the integrin receptors, to the MC3T3-E1 cells, leading to enhanced cellular proliferation.
Keywords: mechano-stimulation, deformable nano-bioactive surfaces, silastic membrane, mc3t3-e1 osteoblast-like cells, cellular proliferation
ECM, extra-cellular matrix; FN, fibronectin; backscattering spectrometry (RBS), AFM, atomic force microscopy; CAG, contact angle goniometry; CM, confocal microscopy
Nano structured bioactive substrates1 are capable of enhancing the proliferation of osteoblast cells. The type and nature of modified surfaces have contributed to the interfacial adhesion of bone cells to bioactive surfaces. Bone cells are known to be affected by mechanical changes in their physiological environment2 through molecular adhesion to the extracellular matrix (ECM) protein. Cells and tissues are constantly under mechanical strains within the physiological environment, however, little is known about the effect of in vivo mechano-transduction strains on cell development and function.3 In this study, the authors focus on the theoretical consideration of the effect of mechano-stimulation on the proliferation of osteoblast-like cells. This fundamental study is dedicated to examining the type of applied mechano-stimulus and its effect on bone cells attached to modified nano-bioactive surfaces.
When bone cells are subjected to mechanical strains, under physiological conditions, minimal cellular strains are believed to occur.4 Studies have reported that strain rates experienced by bone cells gave rise to the formation of bones5 and that the transmission of mechanical forces to the cells was via the ECM proteins.6,7 In data published recently, the authors suggested that cell surface-ECM receptors, called integrins, were responsible for mechano-transduction, since they acted as mechano-receptors.8 In another study that examined the effect of mechano-sensitivity on human bone-derived cells, the authors showed that the human osteoblast-like cells proliferated in response to cycle number and frequency.9 A more recent study5 also reported that, under physiological conditions, growth of osteoblast-like cells and early gene expressions are induced upon mechanical stimulation at short periods of time.
Systems used in the past to study the mechano-stimulation of bone cells included controlling the delivery of hydrostatic pressure as a mechanical input; strain on a substrate; and shear stress caused by a fluid.10 Certain complex systems were designed to produce non-quantitative data and these systems comprised of mechanical inputs with the characteristics of homogeneity and varied degrees of precision. These systems were used in early studies of mechano-transduction in cellular-surfaces.
In past mechano-stimulus studies, the various systems designed included fluid shear stress systems, axial compression systems, longitudinal mechanical strains, hydrostatic pressure systems and out-of-plane mechanical systems. In other studies, special purpose-built systems were made where the combined effect of the deformation of a surface and the application of a fluid stress was studied concurrently.11 Some authors recently confirmed that cellular survival and tissue maintenance was hinged on cell mechano-stimulation, however the mechanism by which cells responded to mechano-stimulation was not clearly understood, since it involved a complexity of mechano-sensors and mechanical strain systems.12 In this study, however, a mechanical system capable of deforming a bioactive nano-structured surface was engineered to investigate the enhanced proliferation of bone cells attached onto a nano-bioactive membrane surface. The study aimed to ascertain whether a novel and improved mechanical system is capable of providing mechano-stimulus to a chemically modified nano-structured membrane surface in order to affect the architecture of bone cell cytoskeleton.
Theoretical introduction
The tissues and organs of the human body experience forces of mechanics on them. These forces are known to act at different levels as well as on the different cells, tissues and organs in the body. Mechanical forces which act through applied loads on hard and soft tissues tend to be strength and mass enhancing. The excessive application of these loads, however, affects the growth and development of the body tissues.13 The different types of human cells responsive to mechano-stimulation, referred to as mechano-sensitive cells include the tenocytes in tendons, fibroblasts in ligaments and skin, osteocytes/osteoblasts in bones, chondrocytes in articular cartilage, and endothelial cells in blood vessels.13
Researchers have designed and developed in vitro systems, in the past, that were used to apply mechanical forces to biological systems in order to study cellular responses. Uniaxial or biaxial tensile stresses have been applied to deformable substrates seeded with cells. Biaxial stretching systems have been devised, which typically use circular elastic membranes to produce isotropic strains independent of stretching direction.14–16 In addition to tensile forces, compressive forces can also be applied to cells that are subjected to compression in vivo, through the application of hydrostatic pressure.17,18
Compressive loading systems can be used to investigate mechano-biological responses of cells in tissues primarily subjected to compression in vivo. The mechano-stimulus systems described provided a lot of insightful information on cellular mechano-biological responses. There are, however, certain limitations associated with such systems since they have not been capable of modeling an in vivo tissue environment in which cells are surrounded by extracellular matrix. As a result, 3-D systems have been developed that enable cells to reside in a more in vivo-like environment, which tend to preserve cell phenotype. External mechanical forces have also been applied to cell-populated collagen gels (CPCGs) to study cellular mechano-biological responses.19,20 Similarly, bio-artificial tissues (BATs) have been developed to embed tendon cells in collagen gels21 where the phenotype of tendon cells in BATs are reported to be better preserved than in 2-D systems where cells are attached to 2-D substrates.22
Internal mechanical forces
External mechano-stimulus is reported to generate internal cellular mechanical forces and these forces are usually referred to as intracellular tension.7,23 The forces are transmitted to the ECM via focal adhesions24 and are referred to as cell traction forces (CTFs). CTFs are central in cellular mechano-biology, since they are directly involved in ECM assembly25 cell shape control26–28 cellular movement29–32 and the maintenance of cellular tensional homeostasis.33,34 They also deform the ECM network by causing stress and strain in the network which in turn modulates cellular functions like gene expression and protein secretions.35,36 CTFs are therefore, critical in many fundamental biological processes like embryogenesis, angiogenesis, and wound healing.2 Mechano-biological investigations, generally, depend on cellular-substrate interactions in order to transmit external mechanical stimuli to cells, since applied mechano-stimulus on cells tend to affect the internal equilibrium state thereby affecting cellular mechano-biological responses.37
Cellular response to mechano-stimulus
The application of mechanical strain to cells substrates influence a number of cellular functions such as proliferation, differentiation, gene expression, protein synthesis of ECM components and the production of cytokines and growth factors. In one study, there was increased proliferation as well as gene expression and protein production of type I collagen in a stretch magnitude-dependent manner of human tendon fibroblasts;38 while another study reported that the repetitive stretching of human tendon fibroblasts at a magnitude of 5% and a frequency of 1 Hz, for one day, increased cell proliferation significantly.
Recent studies39–41 have focused on investigating mechano-biological responses of stem cells and data from such studies show that mechanical forces regulate proliferation and differentiation of stem cells. That is, various mechanical loads applied to bovine bone marrow stem cells (BMSCs) induced differentiation of the stem cells in different cell lineages, including ligament cells, chondrocytes, myocardial and vascular cells. Mechanical conditioning involving the development of systems that applied mechano-stimulus to cell-seeded substrates was used in tissue engineering to enable the development and functioning of tissue replacement constructs in load-bearing tissues/organs in vivo.42,43
Mechano-transduction in cells
Cellular response to mechano-stimulus, in vivo, occurs when applied mechanical strains are converted into chemical signals within the cells which lead to the generation of cellular and molecular events. The process is referred to as mechanotransduction of cells. It follows, however, that the externally applied mechanical strains, acting on the ECM, transmits into the cells via integrin-mediated adhesions44,45 since the primary adhesive receptors and mechano-transducers linking the cellular cytoskeleton to the ECM, are the integrins.23,46 The ECM-integrin-cytoskeleton pathway therefore, plays a key function in the mechano-signaling process, since the applied mechanical strains to the cellular membrane are transmitted immediately and directly to the nucleus through the inter-connected cytoskeleton made up of the microtubules, actin filaments and intermediate filaments.47 Data supporting this model, indicated that the application of mechanical stress to integrins altered the cytoskeleton and activated gene expression in a stress-dependent manner.48,49 Guanine nucleotide-binding proteins (G proteins) have also been reported to function as mechano-transduction molecules, besides integrins.50,51 Increased levels of the intracellular Ca2+ ions (serving as secondary messengers) has been reported to occur, after applying mechanical strains to fibroblasts and other similar cell types.52,53 In addition, recent studies have defined the role of primary cilia in cellular (bone cells) mechanotransduction.54
The mechanisms involved in cellular mechano-transduction, to date, are not clearly understood. However, data has shown enhanced knowledge and level of understanding of the mechanisms involved in cellular mechano-transduction. The role of the ligands in extracellular matrix protein is reported to sensor the applied strains. In addition to that, changes in conformation of the ECM protein, as a result of the external mechano-stimulus affecting the cytoskeleton, influences the strength of α5β1 integrin in fibronectin.55
Mechano-stimulation is known to induce a wide range of cellular events, including proliferation, differentiation, gene and protein expression by both adult differentiated and stem cells.2,13 That is, mechano-transduction, have been identified to take place in cells that are subjected to mechano-stimulus. The response of cells to these stimuli is the conversion of mechanical forces into biochemical process in the cells and a combination of the two types of mechanical forces are purported to act on the cells, in vivo. The type of cellular mechano-stimulation depends on the cells location.2
In vitro systems have been developed to study cellular mechano-biological responses.10,56 These systems employ the application of mechano-stimulation to cells. In such studies, mechanical stretching was applied to cells (such as fibroblasts, epithelial cells, and SMCs) using deformable elastic substrates (e.g., silicone membrane) coated with extracellular matrix (ECM) proteins for the promotion of cellular attachment.
Mechano-stimulation can be applied to cells through a substrate underlying cellular population, either uniaxially or biaxially. Uniaxial stretching is suitable to mechanically loaded tendons and ligaments cells, since the cells are mainly subjected to uniaxial stretching in vivo because of their alignment with long axis along the tendons or ligaments. Biaxial stretching, which is most suitable for dermal fibroblasts and osteoblasts, on the other hand, are applicable to a substrate in two mutually perpendicular directions as an equibiaxial stretch.
Several biaxial stretching systems with a number of advanced features have been developed to present a mechanical environment for cultured cells. These included input force quantitation, loading parameter controls, and homogenous deformation of a cell population.15,57,58 Cells respond to external mechano-stimulus and in turn undergo cellular contraction, owing to the actin-myosin interactions. This is followed by a transmission of the intracellular contraction to the underlying substrate. The forces on the substrate, CTFs, are essential for cellular migration, maintenance of cell shape, and the generation of mechanical signals.59 CTFs, as a result, are implicated in numerous biological processes such as wound healing, embryogenesis, angiogenesis, and inflammation.60 An advanced insight into how CTFs are regulated and transmitted to the ECM will, therefore, be important in understanding the physiological and pathological processes in organs and tissues.2
Classical theory of elastic membranes deformation
The classical theory of elasticity can be used to predict the strain undergone by a circular-shaped membrane that is subjected to an equi-biaxial strain.61 Elastic deformation does not involve any change in orientation since there is no plastic deformation. The effect of strain on a material is related to boundary conditions and the elastic isotropy of the material. When a thin elastic membrane is deformed, upon the application of equi-biaxial strain, the shear components are zero and the strain tensor Є is given by:
(1)
Where X is a dimensionless parameter. For an isotropic membrane that undergoes deformation under equi-biaxial tension (), the strain ratio is given by:
(2)
In this study, the strain experienced by the nano-bioactive silastic membrane is a function of applied stress. The magnitude of the strain ratio, however, cannot be predicted from the stress ratio based on the phenomenological yield equations alone. A model for the theoretical prediction of the strain path of a polycrystalline material deformed under equi-biaxial tension has been proposed by some authors.65 The strain path according to these authors61 is defined by the laws of successive states of the cumulative strain followed by the elastic membrane.
In biaxial stretching, the imposed stress is defined by:
(3)
whereand coincide with the rolling and transverse directions of the membrane respectively. The imposed stress ratio () is assumed to be constant and the stress path is linear. In equi-biaxial tension, and in equi-biaxial stretching , but the magnitudes of Є11 and Є22 are unknown quantities, that can be determined from the properties of the membrane. Assuming the membrane is made up of N groups of grains, each with volume fraction fg (where g=1 to N) and the orientation {hkl} {uvw}; each grain is assumed to undergo the same strain as a poly material, i.e.
(4)
To maintain equilibrium, the summation of the stresses of the individual grains must be equal to the supposed stress such that:
(5)
is solved for each group of grains by applying the flow rule to an isotropic yield function
referred to as the specimen axes.
Conclusions that can be drawn from the theories discussed61 included the following:
that
Elastic membranes were irradiated for 0 and 30 minutes under UV Ozone conditions, in order to activate the nano-surfaces, after which they were characterized using the following procedures: Rutherford backscattering spectrometry (RBS), atomic force microscopy (AFM) and contact angle goniometry (CAG). Mechano-stimulation in the form of dynamic equi-biaxial strains was applied to the elastic nano-bioactive membrane surfaces seeded with bone (MC3T3-E1) cells. Cellular cytoskeletal reorganization was then evaluated using confocal microscopy (CM).
Characterisation of silastic silicone membranes
The elastic membranes were cut to sizes measuring 6.4 cm in cross-sectional diameter and 0.03cm thickness. The process of characterizing the surfaces involved exposure to 10-30 minutes radiation in UVO zone which gave rise to formation of negatively charged (-OH) hydrophilic surfaces; after which the different surface analyses were carried out. The analyses involved carrying out CAG measurements, using sessile drop method, on the surfaces; AFM techniques, under ambient conditions, to analyse roughness of the surfaces-mean surface roughness (Ra in nanometers, nm) of non-treated and functionalized surfaces; RBS analysis to quantitatively determine the elemental composition and the depth profiles of the common elements (C, Si and O) present on the sample surfaces.
Adsorption of FN and cell culture
The membranes were coated with FN after incubation in a concentration of 2.0µg/mL for 1 hour under 37˚C environment, in order to attain an FN monolayer surface coverage. MC3T3-E1 cells were stored under physiological conditions, that is, maintained in 20 mL DMEM medium, at 37˚C under a 5% humidified atmosphere of CO2. The cells were passaged once in every 7 days and fed with DMEM, every other day.
Cellular response to mechano-stimulus
A mechanical system was designed and fabricated to deform an elastic membrane. It consisted of a device having the capability to generate and reproduce mechano-stimulation. This system was designed to enable mechanical strains to be transduced to cells cultured on the membrane, mimicking physiological conditions in vivo. The mechano-stimulus membrane-cell stretching system was designed and engineered at the Cell Biology & Anatomy Department laboratory, School of Dental Medicine at the University of Pennsylvania, Philadelphia, USA. The system was designed to administer equi-biaxial strain levels between 0.2 and 2.0Hz for 2 hours, similar to what pertains under physiological conditions (of 100% humidity atmosphere).The system was capable of deforming a compliant substrate thereby generating a reproducible mechanical strain. When a 1000Pa load was applied by the system to the deformable membrane, equi-biaxial strain regimes of up to 2000µϵ were produced. Prior to the application of the strain regimes, the MC3T3-E1 osteoblast cells were plated onto the membranes at 105cells/cm2concentrations and incubated for 24 hours. After subjecting the elastic membranes to the uniformly equi-biaxial strain regimes for 2hours, the experiment was terminated and the data obtained was evaluated. A controlled experiment comprising of plating the MC3T3-E1 cells on a functionalized membrane, but not subjected to any mechano-stimulus was also mounted.
Cell staining
The cells actin cytoskeleton was stained by Alexa Fluor 488phalloidin (Thermo Fisher Scientific) which is a high affinity filamentous actin (F-actin) conjugated to a bright, photo stable green-fluorescence. This dye is known to selectively stain F-actin at an excitation/emission of 495/518 nm. The phalloidin binds F-actin with high sensitivity, while the Alexa Fluor 488 provides the green fluorescence of distinctive brightness and photo stability. Similarly, the cell nucleus was stained by Nuclear ID Red DNA stain (ENZO), suitable for live-cell staining. This is a cell permaeable dye designed for use in a range of fluorescence detection technologies, in the discrimination of nucleated cells. Cell staining was done by following the standard protocols in bioassays.
Cellular response to the mechano-stimulus effect was evaluated by visualizing the cell cytoskeleton that was treated with Alexa Fluor 488phalloidin and Nuclear ID Red DNA62,63 stains using Confocal Microscope(CM) at 60X magnification. A photomultiplier tube voltage (PTV) was used to quantify the cellular cytoskeletal reorganization, by determining a plane of maximum fluorescence. In addition to that, one way ANOVA and Scheffe’s test at a 0.05 level of significance were used to analyse the data obtained.
The procedures for treating the silicone membranes were optimized such that their elastic characteristics were preserved. Data obtained on the membranes after they were functionalized, indicated that the changes made to their material characteristics were insignificant. There appeared to be no sign of any alterations on the surfaces of the membranes even after subjecting them to the mechano-stimulation.
The chemical characteristics of the membrane surfaces, on the other hand, were significantly altered. To begin with, the CAG data obtained on the surfaces indicated a steady reduction in the degree of wetness of the membranes. That is, between 10º to 20º degree of wetness was observed on 10 minutes UV irradiated surfaces. The nano-bioactive silastic membrane surfaces became hydrophilic due to the hydroxyl (-OH) groups formation on them. The longer periods of exposure of the membrane surfaces to the radiation did not increase the degree of membrane surface hydrophilicity, implying that equilibrium was probably attained at 10 min irradiation, even after longer times of exposure.
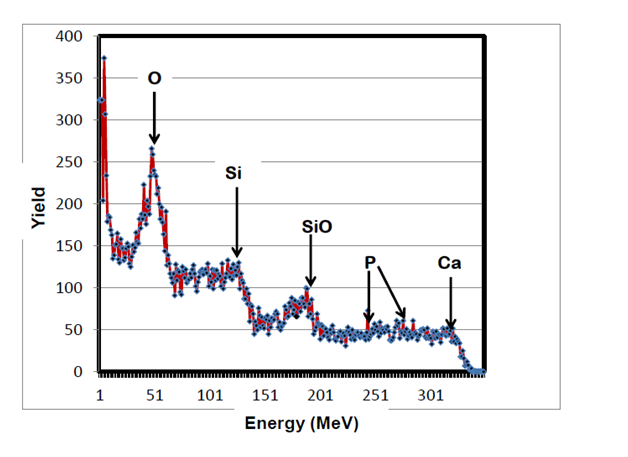
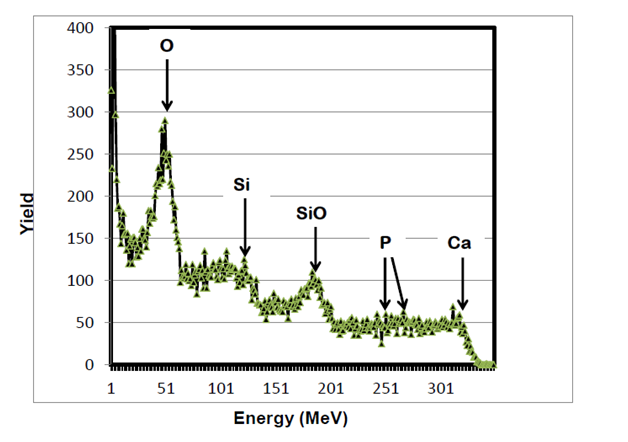
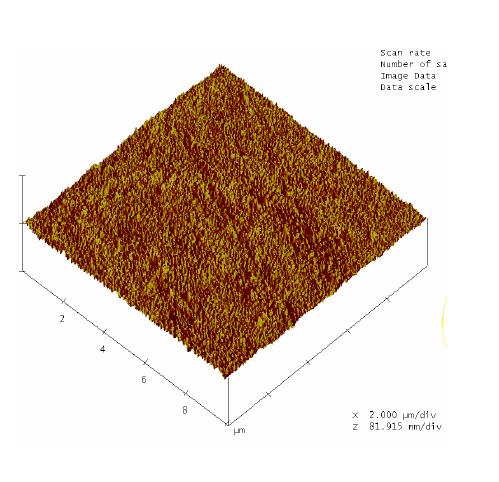
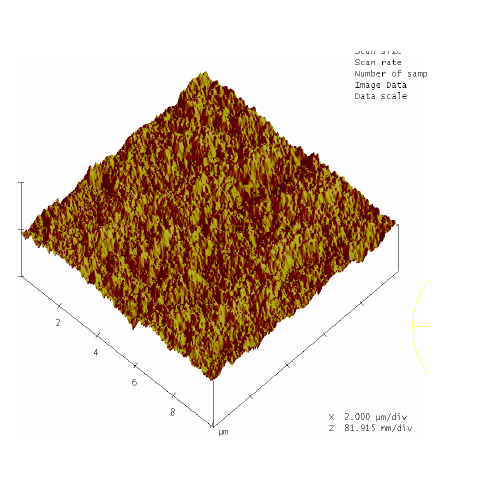
RBS surface analysis showed similarities in the surface profiles of two samples of the nano-bioactive silastic membrane before and after they were exposed to UVO radiation (Figure 1) (Figure 2), while AFM scans/imagery (Figure 3) (Figure 4) clearly portrays the evidence of enhanced roughness on the surface due to irradiation. The root mean square (RMS) values of roughness on the membrane surfaces generated by the AFM system shows a nano-scale monolayer coverage of the ECM protein (FN) in the region of 50 ng/cm2, prior to cellular attachment. One of the advantages of coating the surface of the membranes with FN nano-molecules, besides acting as surface-cellular receptors, was that the nano-molecules significantly reduced the nano-scale surface roughness of the membranes from 6.0 nm to 3.5 nm, as has been reported.64 The reorganization of MC3T3-E1 osteoblasts cellular cytoskeleton was evaluated after applying the dynamic equi-biaxial strain to the membranes, using CM to capture the changes.
Prior to the application of mechano-stimulus, to stretch the silastic membranes, the surfaces were first seeded with the osteoblast-like cells. To distinguish between the cellular cytoskeleton and the nucleus, the cells were treated with Nuclear ID Red DNA (to stain the nucleus) and phalloidin (to stain the cell cytoskeleton) by following a standard procedure for phalloidin staining. The CM images (Figure 5) (Figure 6) displayed an increased level of the fluorescence of the actin filaments as a result of the mechano-stimulus effect. The effect was more noticeable, especially at the cell periphery (Figure 5) (Figure 6). Comparing the two CM images demonstrate that the actin cytoskeleton of the cells grown on the stretched membranes (Figure 6) displayed higher intensity in fluorescence than those on the un-stretched surfaces.
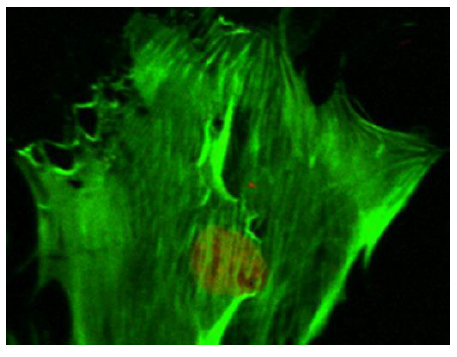
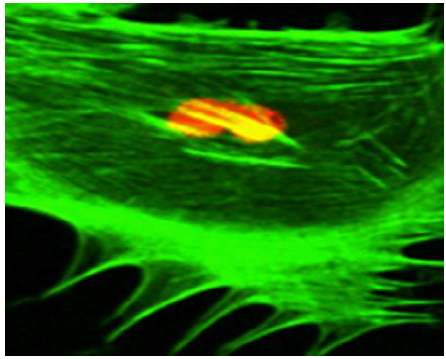
According to the Frost Mechanostatic Theory, when biophysical forces are applied to biological systems under physiological conditions, the forces are translated into cellular response. That is, cellular organisms tend to adapt to their mechanical environment under physiological conditions.
The effect of mechanical stimulus on the functionalized nano-bioactive silicone-surface which was engineered in this study, promoted the proliferation of cellular organisms.65 This was in conformity with data that has been reported in a previous investigation which indicate that, biaxial stretch provides multi-directional stretch both longitudinally and laterally or radially and circumferentially.66 Theoretically, these methods impose equi-biaxial strain on the membrane. It is assumed that with such systems the surfaces are frictionless, and therefore, there are no boundary influences. The biaxial strain provides uniform strain distribution to adherent cells, which helps to comprehend cellular response to applied mechano-stimulation.67 The cell structure is supposed to be controlled by the balanced equibiaxial strains imposed on cell cytoskeleton which enables the cells to withstand, adapt and maintain their physiology in vivo. Most mechano-biological diseases are known to be associated with mechano-transduction between the ECM, the cytoskeleton and the cellular nucleus.68 In addition, cellular structures and cytoskeletal mechanics are known to predict cytoskeletal structure, re-arrangements and cellular responses to strain regimes.69,70 which was evidence in this study.
The findings in this study, however, illustrated that the application of mechano-stimulus to the nano-bioactive elastic membranes were transduced through integrin receptors of the ECM protein, FN, to the cells, resulting in the noticeable changes in the cellular cytoskeletal architecture since higher order mechanical interactions of cell cytoskeletal filaments are influential in determining the mechanical properties of a cell.71 The mechano-stimulus system engineered in this study, therefore, enabled nano-bioactive and nano-structured elastic membrane surfaces to undergo modification in order to achieve linkages to various biomolecules, proteins and the cells. The mechano-stimulus effect on the cell cytoskeleton occurs at the level of the distinct mono mericunits that constitute the filaments of the cytoskeleton. These monomeric units that constitute actin filaments are in the nano scale range; while the molecular mass is in the order of tens of kilo Daltons (≈ x10-24kg).71 The physical properties of these nano-structured actin filaments of the cell cytoskeleton indicate their propensity to respond to mechanical stimulation by adapting to the external stimuli, as visualized in the confocal microscopy images (Figure 5) (Figure 6).
This data further confirms that the proliferation of osteoblast-like bone cells is much more improved on mechanically deformable chemically-modified nano-bioactive membrane surfaces. This may be due to the fact that many of these cellular processes, like cell growth, differentiation, apoptosis, motility, signal transduction and gene expression are known to be driven by, and dependent on, a mechanically intact cytoskeleton to maintain cell shape and structural integrity.67 The study could further be enhanced by further exploring the physico-mechanical mechanisms involved at the cells and the nano-bioactive interfaces, in order to appreciate the cell-surface interactions.
The following people assisted in this study with their technical expertise: they include Doug Yates, Jim Ferris and Kevin Mackee all of Penn Regional Materials Characterization Facility, University of Pennsylvania. This research was partly supported by National Institute of Health [Grant number: ROI-DE-13009].
The author declares no conflict of interest.

©2016 Toworfe, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.