Advances in
eISSN: 2572-8490


Research Article Volume 1 Issue 3
1Laboratory for Transplantation and Regenerative Medicine, Sahlgrenska Academy, University of Gothenburg, Sweden
2Department of Chemistry, Materials and Surfaces–Medical device technology, Sweden
Correspondence: Sumitran Holgersson S, Laboratory for Transplantation and Regenerative Medicine, Sahlgrenska Science Park, Medicinaregatan 8A, 2nd Floor, S-413 46, Gothenburg, Sweden, Tel 46 727490808
Received: October 30, 2016 | Published: December 29, 2016
Citation: Xu B, Håkansson J, Kuna VK, et al. Assessing rat liver-derived biomatrix for hepatic tissue engineering with human fetal liver stem cells. Adv Tissue Eng Regen Med Open Access. 2016;1(3):64–70. DOI: 10.15406/atroa.2016.01.00012
Decellularized organs with preserved architecture and vasculature have been created to produce customized bioengineered organs. Identification of appropriate xenogeneic scaffolds which can support attachment, proliferation and differentiation of human cells is important for studying human organ regeneration. Here, we produced 3-D decellularized scaffolds from rat (n=32) livers preserving the native morphology and vascular structures with complete removal of cellular and nuclear materials. The ability of human liver sinusoidal endothelial cells (LSEC) and two previously well characterized human fetal liver progenitor cell (HFLPC) lines (SV40 and hFL4TERT) to repopulate the a cellular rodent liver tissues was evaluated. We found that, although the major extracellular proteins were preserved, cytokines/growth factors tested were significantly decreased in the decellularized livers. The portal veins were fragile at the end of the decellularization process, resulting in technical problems during recellularization. Even though the vascular network looked preserved after decellularization leakage was observed in majority of the livers. Only in four of the recellularized rat scaffolds attachment of human HFLPC was seen. However, the cells were not evenly distributed in the scaffolds but found scattered as colonies in the rat parenchyma and blood vessels. Approximately 50% of the human cells were viable at the end of recellularization with stable expression of human mitochondria. The cells also showed expression of the endothelial marker L-SIGN and weak expression of human albumin. Few cells expressed hepatocyte-specific markers cytokeratin (CK) 18 and CK8 but, with high variablilty, while but no expression of the biliary cell marker CK19 was found. A few cells expressed CYP3A7 but CYP3A4 was not found. Based on our experience and results we do not believe that decellularized rat liver scaffolds are the ideal scaffolds for reproducible recellularization with human liver stem cells required for studying human liver regeneration or as in vitro models for pharmaceutical toxicity.
Keywords: scaffolds, tissue engineering, regenerative medicine, hepatocyte, chemokines, Matrigel, proteoglycan
LSEC, human liver sinusoidal endothelial cells; HFLPC, human fetal liver progenitor cell; CK, cyto keratin; ECM, extracellular matrix; EDTA, ethylene diamine tetra acetic acid; DL, decellularized liver; GAGs, glycosaminoglycans
The liver is an important organ required for metabolic activities, nutrient storage and detoxification. It is responsible for the production of acute phase proteins, complement components, cytokines and chemokines, and contains large, diverse populations of resident immune cells.1–3 The liver has an enormous capacity to regenerate through different cellular responses depending on the nature and severity of the injury.4 However, when the liver is severely damaged or hepatocyte proliferation is inhibited, the regenerative capacity is overwhelmed resulting in aberrant liver architecture and liver diseases.5,6 This is clinically relevant and results in serious morbidity and mortality.7 To understand the mechanisms of liver diseases, development of three dimensional (3D) models will be useful in order to better recreate cell-cell interactions within their own microenvironment.8,9 Furthermore, the liver is an important target organ for drug testing since all drugs pass through it for detoxification, removing harmful substances from the body. However, conventional in vitro hepatic model systems (i.e. liver slices, cell suspensions, 2-D static monocultures or co-cultures of primary or immortalized hepatocytes) are limited by their inability to maintain histological and phenotypic characteristics over time in culture, due to the non-physiological microenvironment of 2D substrates.10,11 Therefore, to develop safe and effective drugs, 3D cultures of primary human hepatocytes should be used in the early stage of drug development.
Most hepatocyte models in the past have been based on 2D monolayer cultures on surfaces pre-treated with extracellular matrix (ECM) proteins such as collagen, biomatrices, proteoglycan derivatives, soft collagen and Matrigel. While these models have proved invaluable in advancing basic liver biology in both a practical and cost-effective way, they are unable to fully replicate and maintain convincing hepatocyte function in vitro. Human hepatocytes from primary sources cultured in vitro remain viable for only a few days and rapidly deviate from their differentiated phenotype. Maintaining liver parenchymal function ex vivo is especially important as there is need for fully functional hepatocytes to generate stable systems for toxicology screenings and setup of liver disease models.
Relevant, state-of-the-art, organotypic in vitro surrogate systems of human liver and other organs to recreate the multicellular architecture and hemodynamic properties of the organs using novel culture platforms are being developed.12–14 Biologic scaffold materials composed of ECM are typically derived by processes that involve decellularization and subsequent recellularization of tissues or organs 15–17 The ECM of an organ preserves the 3D structure and the original architecture of components such as collagen, elastin, and laminin, allowing the cells to proliferate and differentiate into the specific phenotype for organ bioengineering.18
In recent years, decellularization and recellularization approach for whole organ construction has emerged as exceptionally promising technology.19,20 Use of human fetal liver stem cells which have high proliferative capacity may be an attractive alternative to mature hepatocytes, for whole organ recellularization. Differentiation of stem cells requires the neighboring stem cell niche or microenvironment and extracellular matrix with complete vasculature and integrity. Evidence shows that the differentiation of human hepatic stem cells into mature hepatocytes is more efficient in decellularized liver scaffolds.8,9 Identification of appropriate xenogeneic scaffolds which can support attachment, proliferation and differentiation of human cells is important for studying whole human organ regeneration. We therefore aimed to exploit intact decellularized whole rat livers as scaffolds to differentiate human fetal liver progenitor cells into functional mature hepatocytes.
Retrieval of rat livers
All animal experiments in the present study were performed after prior approval from the local ethics committee for animal studies at the administrative court of appeals in Gothenburg, Sweden. Female Wistar rats, weighing ~250g, were purchased from Taconic, Denmark. The rats were housed in a temperature-controlled (21˚C) facility with a 12-h light-dark cycle with free access to food and water. The animals were acclimatized for at least five days before surgery. The rats were anaesthetized with isoflurane (Isobavet, Shering-Plough Animal Health, Denmark) and placed on their back. The abdominal area was cleaned with ethanol and an incision was made from sternum to the pelvic region. A cannula, connected to a 20ml syringe, was inserted into the portal vein and silk ligatures (4-0, Ethicon, St-Stevens-Woluwe, Belgium) were used to ligate the vein to the cannula. Vena cava inferior and aorta were cut with a pair of scissors caudally to the renal vein and 20ml of heparin/PBS (10IU/ml) was slowly perfused through the portal vein. The syringe was disconnected and the cannula was connected to a peristaltic pump (Ismatec, Germany) and 5mM ethylenediaminetetra acetic acid (EDTA) (Alfa Aesar, Germany) in PBS was perfused through the portal vein at 2ml/min. A cannula was inserted into vena cava superior ~5mm cranially of the right auricle descending into vena cava inferior. Silk ligatures were used to ligate vena cava inferior to the cannula. The hepatic duct, hepatic artery and vena cava inferior (~1cm cranially to the renal vein) were ligated with silk ligatures. To secure the cannulas from slipping out from the portal vein and vena cava superior, respectively, the positions where the cannulas were inserted into the vessels were secured with a drop of tissue glue (Vetbond, 3M, USA). The whole liver was dissected and placed in 5mM EDTA/PBS.
Decellularization protocol
Freshly harvested rat livers were transported from animal room to the laboratory. The cannulated liver portal vein was connected to a peristaltic pump and connected to a degasser (DEGASi, Sweden) via silicon tubing (Figure 1A). All detergents for decellularization were prepared and kept overnight to get rid of air bubbles. The detergents were first passed through the degasser and then through the peristaltic pump into the portal vein of the liver. After thorough rinsing with heparinized PBS, 2% sodium deoxycholate (Sigma, USA) in distilled water was perfused at approximately 2 ml/min for 4 hrs. The scaffold was washed with distilled water (D/W) to remove residual detergent and then perfused with 3% TritonX-100 (Alfa Aesar, Germany) for 4 hrs. The process of decellularization continued until the liver showed an entirely white appearance. Lastly the decellularized liver (DL) was washed continuously for 72 hrs with D/W containing 0.02% sodium azide (Sigma, USA) and 5mM EDTA. The liver was sterilized with 0.1% peracetic acid for 1 hr and once again washed once with 500 ml of sterile PBS. A biopsy was snap frozen in liquid nitrogen and stored at -80˚C and used at a later time point for DNA, collagen, elastin, glycosaminoglycans (GAGs) and cytokines quantification. Another biopsy piece was fixed in 4% formaldehyde (Histolab, Sweden) for immunohistochemistry staining.
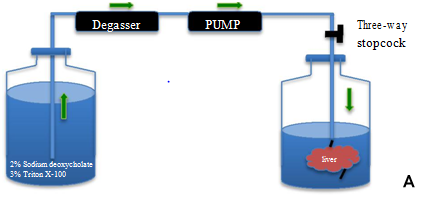


In vitro decellularization of whole rat livers.
Evaluation of vascular bed
To visualize retention of an intact vascular bed, the DLs (n=6) were perfused with Allura Red dye (Sigma, USA). The dye was injected into the portal vein and the structural components of the vascular tree were clearly visualized in the translucent matrix.
DNA quantification
DNA quantification was performed to detect the amount of DNA left after decellularization. Approximately 20 mg of DLs (n=8) and normal liver (n=3) were processed using the DNeasy Blood & Tissue, Qiagen, Germany) according to manufacturer’s instructions. Isolated DNA was quantified using spectrophotometer (Nanodrop, Implen, Germany).
Histological analysis
The DL tissues were fixed in 4% formaldehyde solution (Histolab, Sweden) overnight, dehydrated by tissue processor (Thermo Scientific, Sweden) and embedded in paraffin. The paraffin blocks were sectioned at 5µm thickness and sections were stained using hematoxylin (Histolab, Sweden) and eosin (H&E) (Histolab, Sweden). Collagen fibers were detected by Masson’s trichrome staining (Polysciences, USA). Stained sections were analyzed using a leica microscope (Leica, Germany).
Immunohistochemistry
The DLs were stained for collagen I (1:100, bs-7158R, Bioss, USA), collagen IV (1:200, bs-0806R, Bioss, USA), laminin (1:200, bs-8561R, Bioss, USA) and fibronectin (1:500, ab23751, Abcam, UK). In brief, slides were antigen retrieved in boiling 10mM sodium citrate with 0.05% Tween-20 for 20min. Slides were blocked with serum free protein block (Dako, USA) for 40 min at room temperature and incubated with primary antibodies overnight at 4˚C. After washing in PBS, slides were incubated with secondary antibody (Super picture HRP polymer secondary conjugate, Invitrogen, USA) for 10 min at room temperature. Secondary antibody was visualized using DAB (Dako, USA).
ECM quantification
ECM quantifications of collagen (Sircol S2000, Biocolor, UK), GAGs (Blyscan B1000, Biocolor, UK) and elastin (Fastin F2000, Biocolor, UK) were performed for DLs (n=6 for collagen, n=7 for rest) and normal livers (n=6) using commercial kits.21
Residual cytokine/chemokine determination
The presence of residual cytokines/chemokines after decellularization was quantified using the Luminex technology. Total protein was extracted from DLs (n=3) and normal rat livers (n=3) using the HCYTMAG-60K-PX30 kit (Millipore USA). Luminex was performed according to the manufacturer´s protocol. Total protein was isolated from approximately 30mg of tissue by homogenization for 4 min in tissue rotor at high speed using the 2140 kit (Millipore, Germany). Proteins from DLs were concentrated by lyophilization and the protein amount was measured at 595nm using standard Bradford method (Bio-Rad, USA) in an ELISA reader (Synergy2, Biotek, USA). The protein amount of all tissues was normalized to same concentration with TM buffer (Millipore, Germany) and loaded onto the Luminex plate. The following 30 cytokines/chemokines were tested: EGF, G-CSF, GM-CSF, IFN-α2, IFN -γ, IL-1α, IL-1β, IL-1RA, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12(P40), IL-12(P70), IL-13, IL-15, IL-17A, IP-10, MCP-1, MIP-1α, MIP-1β, TNF-α, TNF-β, VEGF, RANTES, Eotaxin.
Recellularization of livers with human cells
For recellularization of acellular rat liver scaffolds with human liver cells, two different protocols were used. Human liver sinusoidal endothelial cells (LSEC) (Science Cell Research Laboratories, California, USA) and immortalized human fetal liver cells (SV40 and hFL4TERT)22,23 were used for recellularization in both protocols.
Protocol 1
The rat liver was pre-conditioned by perfusion with STEEN solution (Xvivo Perfusion, Sweden) overnight and then in endothelial cell medium (MCDB131 (Gibco, USA), 10% heat inactivated human AB serum (Sigma, USA), 1% L-glutamine (Gibco, UK), EGM-2 single quotes kit except FBS (Lonza, USA), 1% anti-anti (Gibco, USA)) for 2 days at 37˚C. In general, cells were added every second day for 23 days via injection through the portal vein. In first phase (day 1-9) totally 44 million LSEC were added, in second phase (day 13-19) totally 53 million hFL4TERT were added and in last phase (day 20-23) totally 34 million SV40 were added. On day 13, the medium was changed to fetal liver cells medium (DMEM (Lonza, USA), containing heat inactivated 10% human AB serum, 1% L-glutamine, 1% sodium pyruvate (Gibco, China), 1% MEM (Gibco, USA), 30ng/ml HGF, 20ng/ml EGF, 10ng/ml b-FGF, 5ng/ml VEGF, 2ng/ml IL-6, 10ng/ml FGF-9 (all from Peprotech, USA) and 1% anti-anti). Medium was cycled through the liver at 1.5ml/min speed. All perfusions were performed at 37˚C in an incubator supplied with 5% CO2. On day 29, recellularization was stopped; biopsies were taken and fixed in 4% formaldehyde for immunohistochemistry staining.
Protocol 2
The rat liver was pre-conditioned by perfusion with 10µg/ml recombinant collagen (Biocolor, UK) in PBS and 10µg/ml recombinant GAGs (Biocolor, UK) in PBS and incubated at 37˚C for 60 min followed by perfusion with plain DMEM overnight at 37˚C. Medium was then changed to fetal liver cells medium. All three cell types were added continuously in a cycled manner from day 1 to day 7 via portal vein. Totally, 65million LSEC, 44million hFL4TERT and 73million SV40 were added. Medium was cycled through the liver at 0.5ml/min speed. All perfusions were performed at 37˚C in an incubator supplied with 5% CO2. On day 11 liver recellularization was stopped, biopsies were taken and fixed in 4% formaldehyde for immunohistochemistry staining.
Biopsies fixed in 4% formaldehyde were dehydrated, and embedded in paraffin. Tissue sections were deparaffinized and stained with H&E. Immunofluorescence stainings with human specific antibodies to albumin (1:500, A6684, Sigma, USA), CYP3A7 (1:100, ab5540, Abcam, UK), CYP3A4 (1:200, SAB1400064, Sigma, USA), mitochondria (1:100, MAB1273, Millipore, Germany), L-Sign (1:50, SC-17261), CK8 (1:50 SC-8020), CK18 (1:50, SC-51583) and CK19 (1:50, SC-53258) (all from Santa Cruz, USA) were performed. Slides were washed in PBS and incubated with Alexa 488 (Life Technologies, USA) or Alexa 568 (Life Technologies, USA) secondary antibodies for 40 min, co-stained with DAPI (Life Technologies, USA) for 30 seconds and mounted in mounting medium (Dako, USA).
To detect apoptotic cells in recellularized livers, TUNEL staining (Click-iT Plus TUNEL Assay, Life Technologies, USA) was performed in all recellularized livers according to manufacturer’s instructions. The positive control slides (recellularized liver and normal human liver) were treated with DNaseI (Worthington, USA) for 30 min at room temperature before staining.
All values and graphs represent group (decellularized and normal) means. The error bars represent the standard error of the mean. The graphs were plotted using Graph Pad Prism 6 software. The statistical method used was Mann Whitney U test and p<0.05 was considered significant.
Perfusion decellularization of rat livers
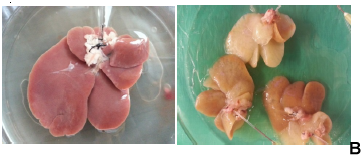
Whole rat liver decellularization was achieved by perfusion of 2% SDC and 3% Triton-X100 and was successfully completed in one cycle of 8 hrs. Although the portal vein was cannulated successfully, at the end of the decellularization process we found that the vein was slender and very fragile. The normal rat livers were pinkish in colour (Figure 1B), and after DC the rat livers became white and translucent (Figure 1C). The vascular network was visualized using Allura Red dye which initially showed that the capillary network was preserved (Figure 1D). However, in many DL the red dye was found diffused through the entire liver bed.
DNA and ECM quantification
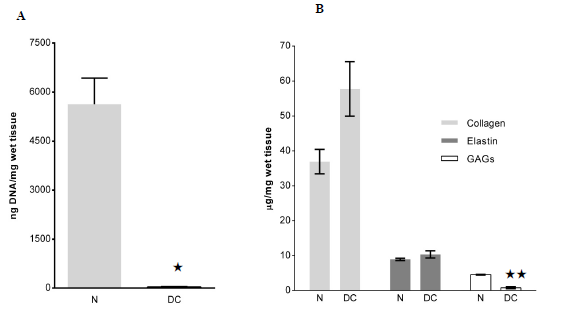
The amount of DNA was significantly decreased in the DL as compared to normal rat liver tissues, 33±32 ng/mg wet tissue and 5629±1387ng/mg respectively (p=0.01; Figure 2A). ECM quantification demonstrated that the amount of GAGs decreased significantly after decellularization. The µg/mg, collagen, GAGs, and elastin contents in the DLs were 57.8±7.8 0.8±0µg/mg. 2µg/mg, and 10 respectively, 4±1.0 as compared to 36.9±3.5(p=0.093), 4.6±0.1(p=0.001) and 9.0±0.3(p=0.073) in normal liver tissue (Figure 2B).

Growth factors determination
Several cytokines and chemokines were found present in the normal rat livers (Figure 2C). However, the amounts of these factors were reduced or below detectable levels in the DLs. In average, 0.1% of original cytokines were retained in DL after decellularization process.
Histological and immunehistochemical examination
H&E staining of the normal rat livers showed presence of intact cells and matrix (Figure 3A), while decellularized liver scaffolds showed absence of cellular material and nuclei (Figure 3B). Masson’s trichrome staining showed the presence of abundant collagen (blue) in the native as well as decellularized scaffolds (Figure 3C) (Figure3D). Immunohistochemical staining of the scaffold confirmed that collagen I, collagen IV, fibronectin and laminin were preserved (brown) in hepatic parenchyma (Figures 3E) (Figure 3H), respectively). The respective negative controls show no positive staining (Figures 3I) (Figure 3L).

Figure 3 Histology and immunohistochemistry of decellularized rat livers. Haematoxylin and Eosin staining of normal (A) and decellularized rat liver (B) showing presence and absence of nucleic material (blue), respectively. Masson’s trichrome staining of normal (C) showing presence of collagen (blue), pink cytoplasm and black nuclei, while the decellularized liver (D) show presence of abundant collagen but absence of cytoplasm and nuclei. Immunohistochemical stainings of decellularized rat livers showing presence of collagen I (E), Collagen IV (F), Fibronectin (G) and Laminin (H). Negative controls (I-L).
Detection of cells in recellularized liver
It is important to mention that of all the recellularized livers analysed, human cells were detected in only 4 livers. Both recellularization protocols gave the same results. Figure 4A shows an example of the gross morphology of the recellularized rat liver with human fetal liver stem cells which looked pinkish in color (due to the presence of cell culture medium). HE staining of the recellularized liver showed that injected human cells were found distributed in the parenchyma (Figure 4B) and also located in the vascular structure (Figure 4C). However, viability of the cells in the various livers was variable at the end of recellularization, as evidenced by TUNEL staining (Figures 5A) (Figure 5D). Immunofluorescence staining with antibodies for human mitochondria showed positive staining in all livers examined confirming recellularized cells were of human origin (Figures 5E). With respect to liver specific markers, a few cells expressed the hepatocyte markers CK18 and CK8, but the expression was highly variable in the livers (Figures 5F) (Figures 5G). Expression of L-SIGN a marker for liver endothelial cells was well demonstrated in the vascular bed of the livers (Figures 5H). Cells were also found to express human albumin in the various livers (Figures 5I), while staining for CYP3A7 was weak and highly variable (Figures 5J) No expression of the biliary epithelial cell marker CK19 was found. Furthermore, CYP3A4 was not observed in any livers (data not shown). Our results in general indicated that the decellularized rat scaffold environment may not be suitable for human liver stem cell differentiation.
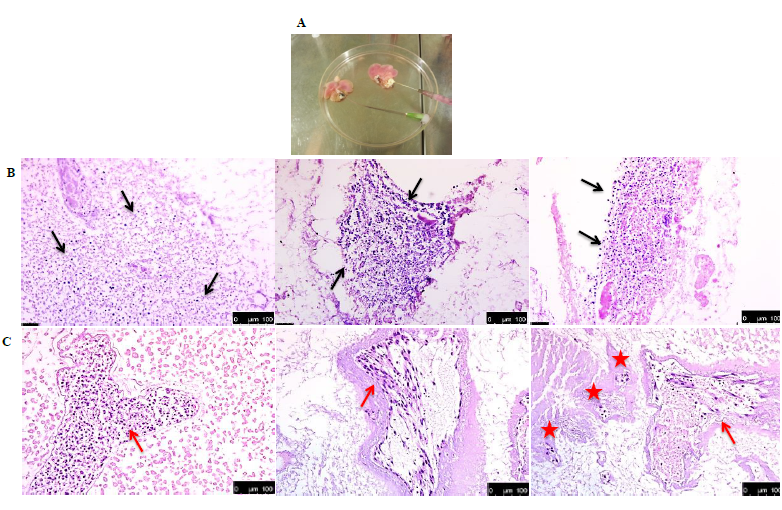
Figure 4 Histology of recellularized rat livers. (A) Gross morphology of recellularized rat livers. The pink colour is due to the culture medium used. Three examples of hematoxylin and eosin staining of rat livers recellularized with human fetal liver stem cells showing presence of cells (blue) distributed in rat parenchyma (black arrows) (B), sinusoids (red asteriks) and blood vessels (red arrows) (C).
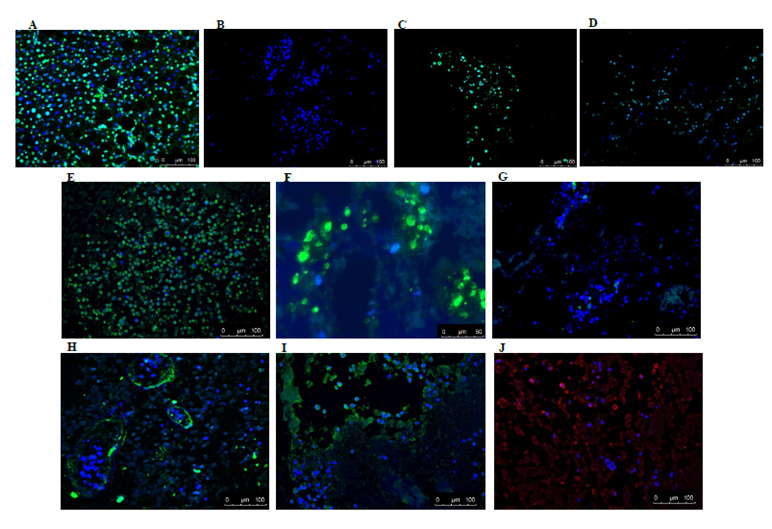
Figure 5 Immunofluorescence staining of recellularized rat livers. TUNEL staining of normal human liver treated with DNaseI (A) shows positive staining (green) for apoptotic cells and living cells (blue nuclei stained with DAPI, while for rat liver scaffolds recellularized with human liver stem cells a great difference in the presence of apoptotic cells was observed between the various livers (B). Representative pictures of positive staining (green) for human specific mitochondrial antigen further corroborated that these cells were of human origin (E). Some of the cells expressed human epithelial cell markers CK18 (green) (F) and CK8 (green) (G). A small portion of cells in recellularized tissue were positive (green) for sinusoidal endothelial marker L- Sign (H), hepatocyte cell marker albumin (green) (I) and fetal liver cell marker CYP3A7 (red) (J), respectively. Nuclei are stained with DAPI (blue).
Biological scaffold-based tissue engineering is an important approach in the field of regenerative medicine. The ECM scaffold is important not only in providing structural support, but also regulating cell attachment, viability and differentiation in engineered organs.24 Here we attempted to recellularize acellular rat liver scaffolds with human fetal liver progenitor cells to test if these scaffolds would support growth and differentiation of these cells. Our results indicate that the decellularization protocol used resulted in successful removal of cellular and nuclear material, preservation of ECM proteins such as collagen and elastin but a significant loss of liver volume and several growth factors/cytokines/chemokines. When introduced into the scaffolds via portal vein, HFLPC attached to the ECM but only in four rat scaffolds, the cells were still viable at the end of recellularization. However, several mature liver markers such as albumin, CK19 and CYP3A4 were weakly or not expressed by the cells, indicating that these scaffolds did not support differentiation of the HFLPC. The most common problem encountered was that the portal veins, which were used for infusion of the decellularization solutions, became very fragile and weak at the end of the process and the diffusion leakage resulting in technical difficulties during recellularization. Several attempts to improve recellularization by pretreatment of scaffolds with collagen prepared from human placenta and varying concentrations of cells were however not successful (unpublished data). Thus, based on our experience and results, we believe that decellularized rat liver scaffolds are unsuitable as scaffolds for recellularization with human liver cells and therefore may not be suitable in vitro models to elucidate human liver regeneration, pathophysiology or pharmaceutical toxicity.
Xenogeneic decellularized rodent, porcine and primate liver scaffolds have become common regenerative models of human tissue.25–27 Although not without some areas for possible improvement, techniques of decellularization are quite mature, however studies focused on re-cellularization strategies and stem cell differentiation are very few. For example, there are only two reports published that have studied the differentiation of human liver stem cells injected via the portal vein into decellularized using rat livers. In Baptista et al.28 reported successful recellularization of rat liver with human fetal liver cells. They were able to create fully functional bioengineered livers by perfusion decellularization of rat livers which were then perfused with human fetal liver and endothelial cells through the vasculature. They observed that the entire area throughout the liver scaffold was repopulated by engrafting into their reputed usual locations. The cells within the repopulated liver displayed typical hepatic, endothelial and biliary epithelial markers.28 Similarly, a recent report by Navarro-Tableros et al.29 demonstrated that human liver stem cells differentiated into mature hepatocytes when injected into acellular rat liver scaffolds. They found that the stem cells lost their embryonic markers and expressed albumin, CK19 and three subtypes of cytochrome P450. Thus, they concluded that the rat ECM provides a favourable environment for differentiation of human liver stem cells.29
Based on the success of the above studies, we explored the potential of acellular whole rat livers as scaffolds for regeneration with human fetal liver cells since 1) the native rat ECM would represent the correct and desired substrate for liver regeneration, 2) three-dimensional acellular liver scaffolds would retain the three-dimensional macrostructure and native micro vascular network, and 3) optimal delivery of nutrients for the regeneration of a fully functional liver. The recellularization results demonstrated successful adhesion of human cells at end of recellularization. This finding is in agreement with the fact that the adhesion process is dependent on the presence of structural matrix proteins such as collagen, fibronectin and laminin, which were still found to be expressed in the decellularized rat livers. However, myriad proteins (e.g., growth factors and hormones, cytokines and chemokines) are known to bind to the matrix and to specific sulfation patterns in the GAGs or to other matrix components.30 Thus the significant loss of GAGs after decellularization of the rat livers, explains the lack of detectable levels of all the tested cytokines and chemokines in the decellularized as compared to native livers. Therefore, it is likely that the loss of important growth factors and chemokines/cytokines may be an important factor contributing to the lack of differentiation of the human fetal liver cells to mature hepatocytes in rat livers. The discrepancy between our findings and those of Baptista et al.28 and Navarro-Tableros et al.29 cannot be explained on the basis of the decellularization process as we have used a standardized protocol commonly used by others. The lack of soluble factors in decellularized rat livers is supported by data from Li et al.31 who demonstrate using proteomic analysis that compared with decellularized human lung, decellularized rat liver has higher collagen and proteoglycan content, but proportionally lower abundance of ECM glycoproteins and no secreted factors. It is important to keep in mind that there are innate differences in the matrix composition, architecture and growths factors between different species which may differentially affect human cell behavior. A recent paper by Balestrini et al.32 shows that there is divergent matrix conservation with respect to species. They compared the biology of decellularized matrix taken from different species; human, primate, pig and rat and found a significant loss of ECM in pig and rat decellularized matrices as compared to human and primate using the same decellularization protocol. They also found that human cells demonstrated preference of human or primate tissues over rat and pig tissue with respect to attachment, proliferation, and health. Thus, it is likely that species-dependent native cues inherent to liver ECM may have profound effects on the suitability of some xenogeneic scaffolds for human liver regeneration.
We conclude that our results demonstrate the feasibility of decellularized whole rat liver tissues in achieving the adhesion of human fetal liver cells; however, they may not be ideal for the differentiation of these cells. Further studies to test whether lack of glycoaminoglycans and important secreted factors such as various growth factors, cytokines, chemokines in decellularized matrices play a significant role in the maturation of engrafted cells are warranted.
This study was financed by the Swedish Government LUA ALF grant, The Lars Erik Gelins foundation, The IngaBritt and Arne Lundbergs foundation and the Swedish Medical Council K2013-65X-22347-01-3 to SSH.
SSH holds shares in Nova Hep AB, a company developing hepatocyte-like cell lines for diagnostic and therapeutic purposes. The other authors have no conflicts of interest.

©2016 Xu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.